Difference between revisions of "RsbS"
| Line 1: | Line 1: | ||
| − | + | scaffold protein of the [[stressosome]], anti-[[RsbT]] | |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || anti-[[RsbT]] | + | |style="background:#ABCDEF;" align="center"| '''Product''' || scaffold protein of the [[stressosome]], anti-[[RsbT]] |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || control of [[SigB]] activity | |style="background:#ABCDEF;" align="center"|'''Function''' || control of [[SigB]] activity | ||
| Line 39: | Line 39: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 86: | Line 82: | ||
* '''Domains:''' | * '''Domains:''' | ||
| − | * '''Modification:''' phosphorylation on Ser-59 [http://www.ncbi.nlm.nih.gov/sites/entrez/17218307 PubMed], dephosphorylation by [[RsbX]] {{PubMed|21362065}} | + | * '''Modification:''' phosphorylation on Ser-59 by [[RsbT]] [http://www.ncbi.nlm.nih.gov/sites/entrez/17218307 PubMed], dephosphorylation by [[RsbX]] {{PubMed|21362065}} |
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| Line 119: | Line 115: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rsbS_520237_520602_1 rsbS] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rsbS_520237_520602_1 rsbS] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/8002610 PubMed] | + | * '''[[Sigma factor]]:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/sites/entrez/8002610 PubMed] |
* '''Regulation:''' constitutively expressed {{PubMed|20019076}} | * '''Regulation:''' constitutively expressed {{PubMed|20019076}} | ||
| Line 154: | Line 150: | ||
==Original Articles== | ==Original Articles== | ||
| − | '''Additional publications:''' {{PubMed|21362065}} | + | '''Additional publications:''' {{PubMed|23320651,21362065}} |
<pubmed>8002610,8682769,9786195,8682789,10781545, 15583165,8824586,10329124,17158665, 21821766 16321960,10671474,8808936,15312768,11244072,15342582,,16321960, 8955331, 12950928, 15466036, 9179850, 18832644 ,17218307 20019076</pubmed> | <pubmed>8002610,8682769,9786195,8682789,10781545, 15583165,8824586,10329124,17158665, 21821766 16321960,10671474,8808936,15312768,11244072,15342582,,16321960, 8955331, 12950928, 15466036, 9179850, 18832644 ,17218307 20019076</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 12:19, 5 February 2013
scaffold protein of the stressosome, anti-RsbT
| Gene name | rsbS |
| Synonyms | ycxS |
| Essential | no |
| Product | scaffold protein of the stressosome, anti-RsbT |
| Function | control of SigB activity |
| Gene expression levels in SubtiExpress: rsbS | |
| Interactions involving this protein in SubtInteract: RsbS | |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 13 kDa, 4.14 |
| Gene length, protein length | 363 bp, 121 aa |
| Immediate neighbours | rsbR, rsbT |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed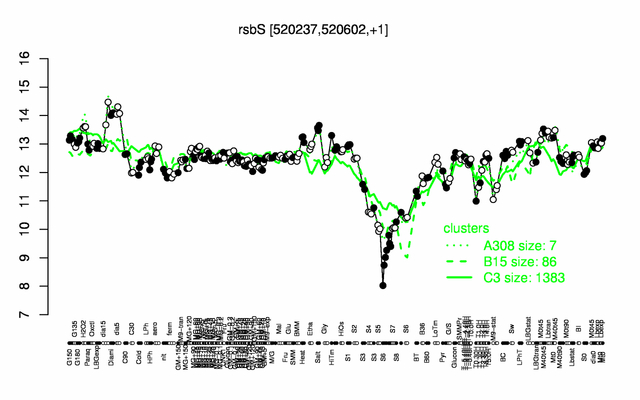
| |
Contents
Categories containing this gene/protein
sigma factors and their control, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU04680
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
- A mutation was found in this gene after evolution under relaxed selection for sporulation PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 3VY9 (complete stressosome)
- UniProt: P42410
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation: constitutively expressed PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Bill Haldenwang, San Antonio, USA
- Chet Price, Davis, USA homepage
- Rick Lewis, Newcastle, UK homepage
Your additional remarks
References
Reviews
Original Articles
Additional publications: PubMed
Christopher T Brown, Laura K Fishwick, Binna M Chokshi, Marissa A Cuff, Jay M Jackson, Travis Oglesby, Alison T Rioux, Enrique Rodriguez, Gregory S Stupp, Austin H Trupp, James S Woollcombe-Clarke, Tracy N Wright, William J Zaragoza, Jennifer C Drew, Eric W Triplett, Wayne L Nicholson
Whole-genome sequencing and phenotypic analysis of Bacillus subtilis mutants following evolution under conditions of relaxed selection for sporulation.
Appl Environ Microbiol: 2011, 77(19);6867-77
[PubMed:21821766]
[WorldCat.org]
[DOI]
(I p)
Adam Reeves, Luis Martinez, William Haldenwang
Expression of, and in vivo stressosome formation by, single members of the RsbR protein family in Bacillus subtilis.
Microbiology (Reading): 2010, 156(Pt 4);990-998
[PubMed:20019076]
[WorldCat.org]
[DOI]
(I p)
Jon Marles-Wright, Tim Grant, Olivier Delumeau, Gijs van Duinen, Susan J Firbank, Peter J Lewis, James W Murray, Joseph A Newman, Maureen B Quin, Paul R Race, Alexis Rohou, Willem Tichelaar, Marin van Heel, Richard J Lewis
Molecular architecture of the "stressosome," a signal integration and transduction hub.
Science: 2008, 322(5898);92-6
[PubMed:18832644]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Adam Reeves, W G Haldenwang
Isolation and characterization of dominant mutations in the Bacillus subtilis stressosome components RsbR and RsbS.
J Bacteriol: 2007, 189(5);1531-41
[PubMed:17158665]
[WorldCat.org]
[DOI]
(P p)
Shuyu Zhang, Adam Reeves, Robyn L Woodbury, W G Haldenwang
Coexpression patterns of sigma(B) regulators in Bacillus subtilis affect sigma(B) inducibility.
J Bacteriol: 2005, 187(24);8520-5
[PubMed:16321960]
[WorldCat.org]
[DOI]
(P p)
Shrin Kuo, Shuyu Zhang, Robyn L Woodbury, W G Haldenwang
Associations between Bacillus subtilis sigmaB regulators in cell extracts.
Microbiology (Reading): 2004, 150(Pt 12);4125-36
[PubMed:15583165]
[WorldCat.org]
[DOI]
(P p)
Chien-Cheng Chen, Michael D Yudkin, Olivier Delumeau
Phosphorylation and RsbX-dependent dephosphorylation of RsbR in the RsbR-RsbS complex of Bacillus subtilis.
J Bacteriol: 2004, 186(20);6830-6
[PubMed:15466036]
[WorldCat.org]
[DOI]
(P p)
Tae-Jong Kim, Tatiana A Gaidenko, Chester W Price
In vivo phosphorylation of partner switching regulators correlates with stress transmission in the environmental signaling pathway of Bacillus subtilis.
J Bacteriol: 2004, 186(18);6124-32
[PubMed:15342582]
[WorldCat.org]
[DOI]
(P p)
Tae-Jong Kim, Tatiana A Gaidenko, Chester W Price
A multicomponent protein complex mediates environmental stress signaling in Bacillus subtilis.
J Mol Biol: 2004, 341(1);135-50
[PubMed:15312768]
[WorldCat.org]
[DOI]
(P p)
Chien-Cheng Chen, Richard J Lewis, Robin Harris, Michael D Yudkin, Olivier Delumeau
A supramolecular complex in the environmental stress signalling pathway of Bacillus subtilis.
Mol Microbiol: 2003, 49(6);1657-69
[PubMed:12950928]
[WorldCat.org]
[DOI]
(P p)
S Zhang, J M Scott, W G Haldenwang
Loss of ribosomal protein L11 blocks stress activation of the Bacillus subtilis transcription factor sigma(B).
J Bacteriol: 2001, 183(7);2316-21
[PubMed:11244072]
[WorldCat.org]
[DOI]
(P p)
J M Scott, J Ju, T Mitchell, W G Haldenwang
The Bacillus subtilis GTP binding protein obg and regulators of the sigma(B) stress response transcription factor cofractionate with ribosomes.
J Bacteriol: 2000, 182(10);2771-7
[PubMed:10781545]
[WorldCat.org]
[DOI]
(P p)
J M Scott, T Mitchell, W G Haldenwang
Stress triggers a process that limits activation of the Bacillus subtilis stress transcription factor sigma(B).
J Bacteriol: 2000, 182(5);1452-6
[PubMed:10671474]
[WorldCat.org]
[DOI]
(P p)
T A Gaidenko, X Yang, Y M Lee, C W Price
Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the environmental stress signaling pathway of Bacillus subtilis.
J Mol Biol: 1999, 288(1);29-39
[PubMed:10329124]
[WorldCat.org]
[DOI]
(P p)
C M Kang, K Vijay, C W Price
Serine kinase activity of a Bacillus subtilis switch protein is required to transduce environmental stress signals but not to activate its target PP2C phosphatase.
Mol Microbiol: 1998, 30(1);189-96
[PubMed:9786195]
[WorldCat.org]
[DOI]
(P p)
S Akbar, C M Kang, T A Gaidenko, C W Price
Modulator protein RsbR regulates environmental signalling in the general stress pathway of Bacillus subtilis.
Mol Microbiol: 1997, 24(3);567-78
[PubMed:9179850]
[WorldCat.org]
[DOI]
(P p)
U Voelker, A Voelker, W G Haldenwang
The yeast two-hybrid system detects interactions between Bacillus subtilis sigmaB regulators.
J Bacteriol: 1996, 178(23);7020-3
[PubMed:8955331]
[WorldCat.org]
[DOI]
(P p)
X Yang, C M Kang, M S Brody, C W Price
Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor.
Genes Dev: 1996, 10(18);2265-75
[PubMed:8824586]
[WorldCat.org]
[DOI]
(P p)
U Voelker, A Voelker, W G Haldenwang
Reactivation of the Bacillus subtilis anti-sigma B antagonist, RsbV, by stress- or starvation-induced phosphatase activities.
J Bacteriol: 1996, 178(18);5456-63
[PubMed:8808936]
[WorldCat.org]
[DOI]
(P p)
C M Kang, M S Brody, S Akbar, X Yang, C W Price
Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor sigma(b) in response to environmental stress.
J Bacteriol: 1996, 178(13);3846-53
[PubMed:8682789]
[WorldCat.org]
[DOI]
(P p)
A Dufour, U Voelker, A Voelker, W G Haldenwang
Relative levels and fractionation properties of Bacillus subtilis σ(B) and its regulators during balanced growth and stress.
J Bacteriol: 1996, 178(13);3701-9 sigma
[PubMed:8682769]
[WorldCat.org]
[DOI]
(P p)
A A Wise, C W Price
Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor sigma B in response to environmental signals.
J Bacteriol: 1995, 177(1);123-33
[PubMed:8002610]
[WorldCat.org]
[DOI]
(P p)