Difference between revisions of "SrfAB"
| Line 1: | Line 1: | ||
| − | + | ||
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || antibiotic synthesis | |style="background:#ABCDEF;" align="center"|'''Function''' || antibiotic synthesis | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU03490 srfAB] | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 400 kDa, 4.903 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 400 kDa, 4.903 | ||
Revision as of 15:00, 6 August 2012
| Gene name | srfAB |
| Synonyms | comL |
| Essential | no |
| Product | surfactin synthetase / competence |
| Function | antibiotic synthesis |
| Gene expression levels in SubtiExpress: srfAB | |
| MW, pI | 400 kDa, 4.903 |
| Gene length, protein length | 10761 bp, 3587 aa |
| Immediate neighbours | srfAA, comS |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed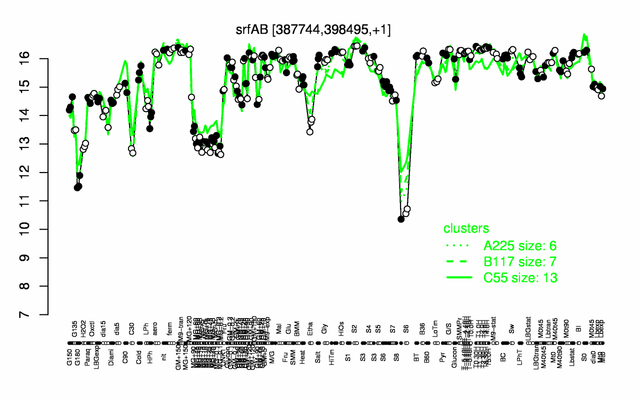
| |
Contents
Categories containing this gene/protein
miscellaneous metabolic pathways, biosynthesis of antibacterial compounds, membrane proteins, phosphoproteins
This gene is a member of the following regulons
Abh regulon, CodY regulon, ComA regulon, PerR regulon, Spx regulon
The gene
Basic information
- Locus tag: BSU03490
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ATP-dependent AMP-binding enzyme family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity:
- Localization: membrane PubMed
Database entries
- Structure:
- UniProt: Q04747
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Additional publications: PubMed