Difference between revisions of "AtpH"
(→Categories containing this gene/protein) |
|||
| Line 74: | Line 74: | ||
* '''Modification:''' | * '''Modification:''' | ||
| + | ** phosphorylated on Arg-159 {{PubMed|22517742}} | ||
* '''Cofactor(s):''' | * '''Cofactor(s):''' | ||
| Line 130: | Line 131: | ||
=References= | =References= | ||
| − | <pubmed>7961438,,16479537</pubmed> | + | <pubmed>7961438,22517742,16479537</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:06, 21 April 2012
- Description: ATP synthase (subunit delta)
| Gene name | atpH |
| Synonyms | |
| Essential | no |
| Product | ATP synthase (subunit delta)) |
| Function | ATP synthesis |
| MW, pI | 19 kDa, 9.572 |
| Gene length, protein length | 543 bp, 181 aa |
| Immediate neighbours | atpA, atpF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 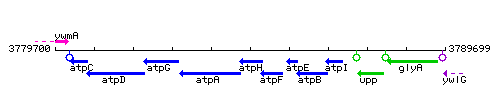
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
ATP synthesis, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU36840
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP synthesis see a video
- Protein family: ATPase delta chain family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-159 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Localization: Membrane (Heterogeneous) PubMed
Database entries
- Structure:
- UniProt: P37811
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
M Santana, M S Ionescu, A Vertes, R Longin, F Kunst, A Danchin, P Glaser
Bacillus subtilis F0F1 ATPase: DNA sequence of the atp operon and characterization of atp mutants.
J Bacteriol: 1994, 176(22);6802-11
[PubMed:7961438]
[WorldCat.org]
[DOI]
(P p)