Difference between revisions of "TrpD"
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || biosynthesis of tryptophan | |style="background:#ABCDEF;" align="center"|'''Function''' || biosynthesis of tryptophan | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU22670 trpD] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/phenyl_tyr_tryp.html Phe, Tyr, Trp]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/phenyl_tyr_tryp.html Phe, Tyr, Trp]''' | ||
Revision as of 11:04, 7 August 2012
- Description: anthranilate phosphoribosyltransferase
| Gene name | trpD |
| Synonyms | |
| Essential | no |
| Product | anthranilate phosphoribosyltransferase |
| Function | biosynthesis of tryptophan |
| Gene expression levels in SubtiExpress: trpD | |
| Metabolic function and regulation of this protein in SubtiPathways: Phe, Tyr, Trp | |
| MW, pI | 35 kDa, 4.904 |
| Gene length, protein length | 1014 bp, 338 aa |
| Immediate neighbours | trpC, trpE |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 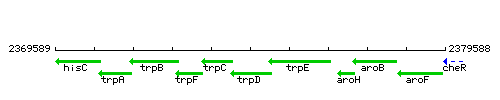
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed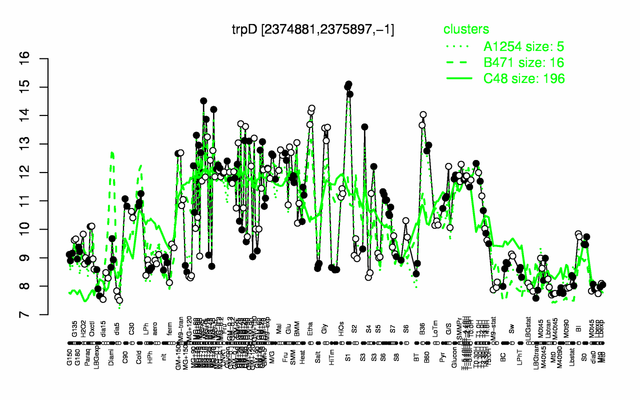
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU22670
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: N-(5-phospho-D-ribosyl)-anthranilate + diphosphate = anthranilate + 5-phospho-alpha-D-ribose 1-diphosphate (according to Swiss-Prot)
- Protein family: 2-oxoacid dehydrogenase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P03947
- KEGG entry: [3]
- E.C. number: 2.4.2.18
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol. 2011 81(6): 1459-1473. PubMed:21815947