Difference between revisions of "HmoB"
| Line 12: | Line 12: | ||
|style="background:#ABCDEF;" align="center"| '''Product''' || heme monooxygenase | |style="background:#ABCDEF;" align="center"| '''Product''' || heme monooxygenase | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || degradation of heme | + | |style="background:#ABCDEF;" align="center"|'''Function''' || degradation of heme, [[acquisition of iron]] |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 18 kDa, 5.216 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 18 kDa, 5.216 | ||
| Line 92: | Line 92: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/explore/explore.do?structureId=3TVZ 3TVZ] {{PubMed|22531134}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P38049 P38049] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P38049 P38049] | ||
| Line 137: | Line 137: | ||
=References= | =References= | ||
'''Additional references:''' {{PubMed|21873409}} | '''Additional references:''' {{PubMed|21873409}} | ||
| − | <pubmed> 8335642 </pubmed> | + | <pubmed> 8335642 22531134 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:30, 27 April 2012
- Description: heme monooxygenase
| Gene name | hmoB |
| Synonyms | yixC, yhgC |
| Essential | no |
| Product | heme monooxygenase |
| Function | degradation of heme, acquisition of iron |
| MW, pI | 18 kDa, 5.216 |
| Gene length, protein length | 498 bp, 166 aa |
| Immediate neighbours | yhgB, pbpF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 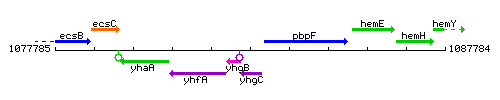
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU10100
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: binds hemin in vitro with ~1:1 stoichiometry and degrade hemin in the presence of an electron donor PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P38049
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- constitutively expressed PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional references: PubMed