Difference between revisions of "KatA"
(→Categories containing this gene/protein) |
|||
| Line 44: | Line 44: | ||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| − | {{SubtiWiki category|[[resistance against oxidative and electrophile stress]]}} | + | {{SubtiWiki category|[[resistance against oxidative and electrophile stress]]}}, |
| + | {{SubtiWiki category|[[phosphoproteins]]}} | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
Revision as of 15:22, 20 April 2012
- Description: main vegetative catalase 1
| Gene name | katA |
| Synonyms | kat-19 |
| Essential | no |
| Product | vegetative catalase |
| Function | detoxification (degradation) of hydrogen peroxide |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 54 kDa, 6.151 |
| Gene length, protein length | 1449 bp, 483 aa |
| Immediate neighbours | senS, ssuB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 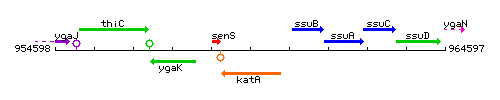
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
resistance against oxidative and electrophile stress, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU08820
Phenotypes of a mutant
- increased sensitivity to oxidative stress PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 H2O2 = O2 + 2 H2O (according to Swiss-Prot)
- Protein family: catalase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): contains an iron-sulfur cluster, heme
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 1SI8 (enzyme from Enterococcus faecalis, 68% identity)
- UniProt: P26901
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: katA PubMed
- Sigma factor:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Melinda J Faulkner, Zhen Ma, Mayuree Fuangthong, John D Helmann
Derepression of the Bacillus subtilis PerR peroxide stress response leads to iron deficiency.
J Bacteriol: 2012, 194(5);1226-35
[PubMed:22194458]
[WorldCat.org]
[DOI]
(I p)
Wang Yung Tu, Susanne Pohl, Pijug Summpunn, Silvio Hering, Sandra Kerstan, Colin R Harwood
Comparative analysis of the responses of related pathogenic and environmental bacteria to oxidative stress.
Microbiology (Reading): 2012, 158(Pt 3);636-647
[PubMed:22174384]
[WorldCat.org]
[DOI]
(I p)
A F Herbig, J D Helmann
Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA.
Mol Microbiol: 2001, 41(4);849-59
[PubMed:11532148]
[WorldCat.org]
[DOI]
(P p)
L Casillas-Martinez, P Setlow
Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents.
J Bacteriol: 1997, 179(23);7420-5
[PubMed:9393707]
[WorldCat.org]
[DOI]
(P p)
S Engelmann, M Hecker
Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE.
FEMS Microbiol Lett: 1996, 145(1);63-9
[PubMed:8931328]
[WorldCat.org]
[DOI]
(P p)
N Bsat, L Chen, J D Helmann
Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes.
J Bacteriol: 1996, 178(22);6579-86
[PubMed:8932315]
[WorldCat.org]
[DOI]
(P p)
L Chen, L Keramati, J D Helmann
Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions.
Proc Natl Acad Sci U S A: 1995, 92(18);8190-4
[PubMed:7667267]
[WorldCat.org]
[DOI]
(P p)