Difference between revisions of "GltB"
| Line 30: | Line 30: | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:gltB_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:gltB_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=gltB_2008572_2010053_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:gltB_expression.png|500px]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | |||
<br/><br/><br/><br/><br/><br/> | <br/><br/><br/><br/><br/><br/> | ||
Revision as of 10:40, 19 April 2012
- Description: small subunit of glutamate synthase
| Gene name | gltB |
| Synonyms | |
| Essential | no |
| Product | glutamate synthase (small subunit) |
| Function | glutamate biosynthesis |
| Interactions involving this protein in SubtInteract: GltB | |
| Metabolic function and regulation of this protein in SubtiPathways: Ammonium/ glutamate | |
| MW, pI | 54.6 kDa, 7.69 |
| Gene length, protein length | 1479 bp, 493 amino acids |
| Immediate neighbours | yogA, gltA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 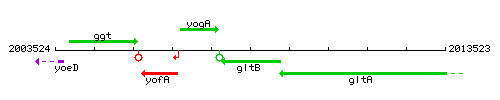
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed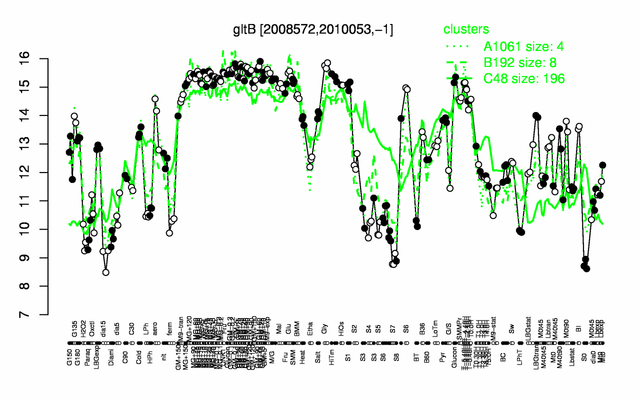
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids
This gene is a member of the following regulons
GltC regulon, FsrA regulon, TnrA regulon
The gene
Basic information
- Locus tag: BSU18440
Phenotypes of a mutant
auxotrophic for glutamate
Database entries
- DBTBS entry: [1]
- SubtiList entry:[2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 L-glutamate + NADP+ = L-glutamine + 2-oxoglutarate + NADPH (according to Swiss-Prot) 2 L-glutamate + NADP(+) <=> L-glutamine + 2-oxoglutarate + NADPH
- Protein family: glutamate synthase family.
- Paralogous protein(s): none
Extended information on the protein
- Kinetic information:
- Domains:
- nucleotide binding domain (NADP) (299–313)
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: O34399
- KEGG entry: [3]
- E.C. number: 1.4.1.13
Additional information
Expression and regulation
- Regulation: see gltA
- Additional information:
Biological materials
- Mutant: GP807 (del gltAB::tet), GP517 (ermC), both available in Stülke lab
- Expression vector:
- lacZ fusion: see gltA
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Linc Sonenshein, Tufts University, Boston, MA, USA Homepage
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
Reviews
Original publications