Difference between revisions of "RnpB"
| Line 8: | Line 8: | ||
|style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''rnaP '' | |style="background:#ABCDEF;" align="center"| '''Synonyms''' || ''rnaP '' | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Essential''' || | + | |style="background:#ABCDEF;" align="center"| '''Essential''' || yes {{PubMed|23109554}} |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''Product''' || RNA component of ribonuclease P <br/>([[RNase]] P) (catalytic subunit, ribozyme) | |style="background:#ABCDEF;" align="center"| '''Product''' || RNA component of ribonuclease P <br/>([[RNase]] P) (catalytic subunit, ribozyme) | ||
| Line 35: | Line 35: | ||
{{SubtiWiki category|[[Rnases]]}}, | {{SubtiWiki category|[[Rnases]]}}, | ||
{{SubtiWiki category|[[translation]]}}, | {{SubtiWiki category|[[translation]]}}, | ||
| − | {{SubtiWiki category|[[ncRNA]]}} | + | {{SubtiWiki category|[[ncRNA]]}}, |
| + | {{SubtiWiki category|[[essential genes]]}} | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| Line 47: | Line 48: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | + | * essential {{PubMed|23109554}} | |
=== Database entries === | === Database entries === | ||
| Line 108: | Line 109: | ||
==Original Publications== | ==Original Publications== | ||
| − | '''Additional publications:''' {{PubMed|21076397,21622899}} | + | '''Additional publications:''' {{PubMed|23109554,21076397,21622899}} |
<pubmed>16470227,17355991,11812129,10390342,11454206, 19549719,11454206, 19717440 ,20133747 19932118 20188108 20434461 17299131 14510496 12951523 12836338 11577704 20557101 </pubmed> | <pubmed>16470227,17355991,11812129,10390342,11454206, 19549719,11454206, 19717440 ,20133747 19932118 20188108 20434461 17299131 14510496 12951523 12836338 11577704 20557101 </pubmed> | ||
[[Category:small RNA]] | [[Category:small RNA]] | ||
Revision as of 13:12, 31 October 2012
- Description: RNA component of RNase P
| Gene name | rnpB |
| Synonyms | rnaP |
| Essential | yes PubMed |
| Product | RNA component of ribonuclease P (RNase P) (catalytic subunit, ribozyme) |
| Function | cleavage of precursor sequences from the 5' ends of pre-tRNAs |
| Interactions involving this RNA in SubtInteract: RnpB | |
| Gene length | 401 bp |
| Immediate neighbours | ypsC, ypsB |
| Gene sequence (+200bp) | |
Genetic context 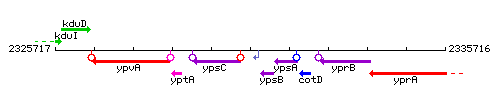
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
Rnases, translation, ncRNA, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag:
Phenotypes of a mutant
- essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
- Structure:
The RNA
- Biological activity: 5' end maturation of precursor tRNAs
- RNA family:
- cofactor: divalent cations (Ca(II) or Mg (II)) for the stabilization of the complex with pre-tRNA PubMed
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/RNA
- Carol Fierke, University of Michigan, Ann Arbor, USA homepage
- Roland Hartmann, Marburg University, Germany homepage
Your additional remarks
References
Reviews
Original Publications
Additional publications: PubMed
Ruiting Liang, Elzbieta Kierzek, Ryszard Kierzek, Douglas H Turner
Comparisons between chemical mapping and binding to isoenergetic oligonucleotide microarrays reveal unexpected patterns of binding to the Bacillus subtilis RNase P RNA specificity domain.
Biochemistry: 2010, 49(37);8155-68
[PubMed:20557101]
[WorldCat.org]
[DOI]
(I p)
John Hsieh, Kristin S Koutmou, David Rueda, Markos Koutmos, Nils G Walter, Carol A Fierke
A divalent cation stabilizes the active conformation of the B. subtilis RNase P x pre-tRNA complex: a role for an inner-sphere metal ion in RNase P.
J Mol Biol: 2010, 400(1);38-51
[PubMed:20434461]
[WorldCat.org]
[DOI]
(I p)
Nathan J Baird, Haipeng Gong, Syed S Zaheer, Karl F Freed, Tao Pan, Tobin R Sosnick
Extended structures in RNA folding intermediates are due to nonnative interactions rather than electrostatic repulsion.
J Mol Biol: 2010, 397(5);1298-306
[PubMed:20188108]
[WorldCat.org]
[DOI]
(I p)
Kristin S Koutmou, Anette Casiano-Negroni, Melissa M Getz, Samuel Pazicni, Andrew J Andrews, James E Penner-Hahn, Hashim M Al-Hashimi, Carol A Fierke
NMR and XAS reveal an inner-sphere metal binding site in the P4 helix of the metallo-ribozyme ribonuclease P.
Proc Natl Acad Sci U S A: 2010, 107(6);2479-84
[PubMed:20133747]
[WorldCat.org]
[DOI]
(I p)
Kristin S Koutmou, Nathan H Zahler, Jeffrey C Kurz, Frank E Campbell, Michael E Harris, Carol A Fierke
Protein-precursor tRNA contact leads to sequence-specific recognition of 5' leaders by bacterial ribonuclease P.
J Mol Biol: 2010, 396(1);195-208
[PubMed:19932118]
[WorldCat.org]
[DOI]
(I p)
Stefanie A Mortimer, Kevin M Weeks
C2'-endo nucleotides as molecular timers suggested by the folding of an RNA domain.
Proc Natl Acad Sci U S A: 2009, 106(37);15622-7
[PubMed:19717440]
[WorldCat.org]
[DOI]
(I p)
John Hsieh, Carol A Fierke
Conformational change in the Bacillus subtilis RNase P holoenzyme--pre-tRNA complex enhances substrate affinity and limits cleavage rate.
RNA: 2009, 15(8);1565-77
[PubMed:19549719]
[WorldCat.org]
[DOI]
(I p)
Barbara Wegscheid, Roland K Hartmann
In vivo and in vitro investigation of bacterial type B RNase P interaction with tRNA 3'-CCA.
Nucleic Acids Res: 2007, 35(6);2060-73
[PubMed:17355991]
[WorldCat.org]
[DOI]
(I p)
Somashekarappa Niranjanakumari, Jeremy J Day-Storms, Mahiuddin Ahmed, John Hsieh, Nathan H Zahler, Ronald A Venters, Carol A Fierke
Probing the architecture of the B. subtilis RNase P holoenzyme active site by cross-linking and affinity cleavage.
RNA: 2007, 13(4);521-35
[PubMed:17299131]
[WorldCat.org]
[DOI]
(P p)
Barbara Wegscheid, Ciarán Condon, Roland K Hartmann
Type A and B RNase P RNAs are interchangeable in vivo despite substantial biophysical differences.
EMBO Rep: 2006, 7(4);411-7
[PubMed:16470227]
[WorldCat.org]
[DOI]
(P p)
Tomoaki Ando, Terumichi Tanaka, Yo Kikuchi
Bacterial ribonuclease P reaction is affected by substrate shape and magnesium ion concentration.
Nucleic Acids Res Suppl: 2003, (3);293-4
[PubMed:14510496]
[DOI]
(P p)
Tomoaki Ando, Terumichi Tanaka, Yo Kikuchi
Comparative analyses of hairpin substrate recognition by Escherichia coli and Bacillus subtilis ribonuclease P ribozymes.
Biosci Biotechnol Biochem: 2003, 67(8);1825-7
[PubMed:12951523]
[WorldCat.org]
[DOI]
(P p)
Y Hori, T Tanaka, Y Kikuchi
In vitro hyperprocessing of tRNAs by Bacillus subtilis ribonuclease P RNA.
Nucleic Acids Res Suppl: 2001, (1);209-10
[PubMed:12836338]
[DOI]
(P p)
Christoph Rox, Ralph Feltens, Thomas Pfeiffer, Roland K Hartmann
Potential contact sites between the protein and RNA subunit in the Bacillus subtilis RNase P holoenzyme.
J Mol Biol: 2002, 315(4);551-60
[PubMed:11812129]
[WorldCat.org]
[DOI]
(P p)
Y Hori, E Sakai, T Tanaka, Y Kikuchi
Hyperprocessing reaction of tRNA by Bacillus subtilis ribonuclease P ribozyme.
FEBS Lett: 2001, 505(2);337-9
[PubMed:11577704]
[WorldCat.org]
[DOI]
(P p)
A Hansen, T Pfeiffer, T Zuleeg, S Limmer, J Ciesiolka, R Feltens, R K Hartmann
Exploring the minimal substrate requirements for trans-cleavage by RNase P holoenzymes from Escherichia coli and Bacillus subtilis.
Mol Microbiol: 2001, 41(1);131-43
[PubMed:11454206]
[WorldCat.org]
[DOI]
(P p)
J M Warnecke, R Held, S Busch, R K Hartmann
Role of metal ions in the hydrolysis reaction catalyzed by RNase P RNA from Bacillus subtilis.
J Mol Biol: 1999, 290(2);433-45
[PubMed:10390342]
[WorldCat.org]
[DOI]
(P p)