Difference between revisions of "PtkA"
(→Original publications) |
|||
| Line 38: | Line 38: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | + | * Accumulation of extra chromosome equivalents [http://www.ncbi.nlm.nih.gov/sites/entrez/17367396 PubMed] | |
| − | Accumulation of extra chromosome equivalents [http://www.ncbi.nlm.nih.gov/sites/entrez/17367396 PubMed] | + | * Defect in biofilm formation, this involves the kinase activity, but the target protein is unknown {{PubMed|20815827}} |
| − | |||
=== Database entries === | === Database entries === | ||
| Line 89: | Line 88: | ||
=Expression and regulation= | =Expression and regulation= | ||
| + | * '''Operon:''' ''[[tkmA]]-[[ptkA]]-[[ptpZ]]-[[ugd]]'' {{PubMed|20815827,12970183}} | ||
| − | * | + | * '''[[Sigma factor]]:''' [[SigA]] {{PubMed|20815827}} |
| − | |||
| − | |||
* '''Regulation:''' | * '''Regulation:''' | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| + | ** [[DegU]]-P: transcription repression {{PubMed|20815827}} | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| Line 123: | Line 122: | ||
<pubmed> 20497498 </pubmed> | <pubmed> 20497498 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed> 12970183, 15741737, 15866923, 17367396, 19258708 18547145 20497499 20509597 </pubmed> | + | <pubmed> 12970183, 15741737, 15866923, 17367396, 19258708 18547145 20497499 20509597 20815827</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:28, 13 September 2010
- Description: protein tyrosine kinase
| Gene name | ptkA |
| Synonyms | ywqD |
| Essential | no |
| Product | protein tyrosine kinase |
| Function | protein phosphorylation |
| MW, pI | 25 kDa, 9.628 |
| Gene length, protein length | 711 bp, 237 aa |
| Immediate neighbours | ptpZ, tkmA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 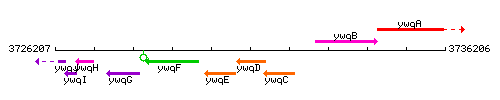
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU36250
Phenotypes of a mutant
- Accumulation of extra chromosome equivalents PubMed
- Defect in biofilm formation, this involves the kinase activity, but the target protein is unknown PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + a [protein]-L-tyrosine = ADP + a [protein]-L-tyrosine phosphate (according to Swiss-Prot), autophosphorylation, phosphorylation of Ugd, TuaD, Ssb, SsbB
- Protein family: cpsD/capB family (according to Swiss-Prot), BY-kinase
- Paralogous protein(s): EpsB
Extended information on the protein
- Kinetic information:
- Domains: single BY-kinase domain
- Modification: autophosphorylation at residues Y225, Y227 and Y228 (primary site) PubMed, dephosphorylated by PtpZ PubMed
- Cofactor(s): ATP
- Effectors of protein activity: TkmA - transmembrane modulator, activates PtkA autophosphorylation and substrate phosphorylation PubMed
- Localization:
Database entries
- Structure: 2VED (CapB, the homolog in Staphylococcus aureus)
- UniProt: P96716
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant: KO strain created with pMUTIN-2, available from Ivan Mijakovic
- Expression vector: pQE-30, N-terminally 6xHis-tagged, available from Ivan Mijakovic
- lacZ fusion: in a KO strain created with pMUTIN-2, available from Ivan Mijakovic
- GFP fusion: CFP-fusion, available from Ivan Mijakovic
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Ivan Mijakovic, Thiverval-Grignon, France
Your additional remarks
References
Reviews
Original publications
Taryn B Kiley, Nicola R Stanley-Wall
Post-translational control of Bacillus subtilis biofilm formation mediated by tyrosine phosphorylation.
Mol Microbiol: 2010, 78(4);947-63
[PubMed:20815827]
[WorldCat.org]
[DOI]
(I p)
Boumediene Soufi, Chanchal Kumar, Florian Gnad, Matthias Mann, Ivan Mijakovic, Boris Macek
Stable isotope labeling by amino acids in cell culture (SILAC) applied to quantitative proteomics of Bacillus subtilis.
J Proteome Res: 2010, 9(7);3638-46
[PubMed:20509597]
[WorldCat.org]
[DOI]
(I p)
Carsten Jers, Malene Mejer Pedersen, Dafni Katerina Paspaliari, Wolfgang Schütz, Christina Johnsson, Boumediene Soufi, Boris Macek, Peter Ruhdal Jensen, Ivan Mijakovic
Bacillus subtilis BY-kinase PtkA controls enzyme activity and localization of its protein substrates.
Mol Microbiol: 2010, 77(2);287-99
[PubMed:20497499]
[WorldCat.org]
[DOI]
(I p)
Dina Petranovic, Christophe Grangeasse, Boris Macek, Mohammad Abdillatef, Virginie Gueguen-Chaignon, Sylvie Nessler, Josef Deutscher, Ivan Mijakovic
Activation of Bacillus subtilis Ugd by the BY-kinase PtkA proceeds via phosphorylation of its residue tyrosine 70.
J Mol Microbiol Biotechnol: 2009, 17(2);83-9
[PubMed:19258708]
[WorldCat.org]
[DOI]
(I p)
Vanesa Olivares-Illana, Philippe Meyer, Emmanuelle Bechet, Virginie Gueguen-Chaignon, Didier Soulat, Sylvie Lazereg-Riquier, Ivan Mijakovic, Josef Deutscher, Alain J Cozzone, Olivier Laprévote, Solange Morera, Christophe Grangeasse, Sylvie Nessler
Structural basis for the regulation mechanism of the tyrosine kinase CapB from Staphylococcus aureus.
PLoS Biol: 2008, 6(6);e143
[PubMed:18547145]
[WorldCat.org]
[DOI]
(I p)
Dina Petranovic, Ole Michelsen, Ksenija Zahradka, Catarina Silva, Mirjana Petranovic, Peter Ruhdal Jensen, Ivan Mijakovic
Bacillus subtilis strain deficient for the protein-tyrosine kinase PtkA exhibits impaired DNA replication.
Mol Microbiol: 2007, 63(6);1797-805
[PubMed:17367396]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Lucia Musumeci, Lutz Tautz, Dina Petranovic, Robert A Edwards, Peter Ruhdal Jensen, Tomas Mustelin, Josef Deutscher, Nunzio Bottini
In vitro characterization of the Bacillus subtilis protein tyrosine phosphatase YwqE.
J Bacteriol: 2005, 187(10);3384-90
[PubMed:15866923]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Dina Petranovic, Josef Deutscher
How tyrosine phosphorylation affects the UDP-glucose dehydrogenase activity of Bacillus subtilis YwqF.
J Mol Microbiol Biotechnol: 2004, 8(1);19-25
[PubMed:15741737]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Sandrine Poncet, Grégory Boël, Alain Mazé, Sylvie Gillet, Emmanuel Jamet, Paulette Decottignies, Christophe Grangeasse, Patricia Doublet, Pierre Le Maréchal, Josef Deutscher
Transmembrane modulator-dependent bacterial tyrosine kinase activates UDP-glucose dehydrogenases.
EMBO J: 2003, 22(18);4709-18
[PubMed:12970183]
[WorldCat.org]
[DOI]
(P p)