Difference between revisions of "Hfq"
(→Original publications) |
(→Original publications) |
||
| Line 145: | Line 145: | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>12850135, 20445260 23457461 22965117,22053080 24932523 25150227 </pubmed> | + | <pubmed>12850135, 20445260 23457461 22965117,22053080 24932523 25150227 25915524</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Latest revision as of 15:41, 29 April 2015
- Description: RNA chaperone
| Gene name | hfq |
| Synonyms | ymaH |
| Essential | no |
| Product | RNA chaperone |
| Function | unknown |
| Gene expression levels in SubtiExpress: hfq | |
| MW, pI | 8 kDa, 8.698 |
| Gene length, protein length | 219 bp, 73 aa |
| Immediate neighbours | miaA, ymzC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 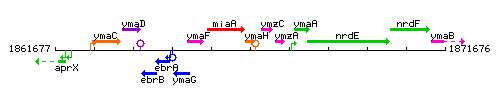
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17340
Phenotypes of a mutant
Database entries
- BsubCyc: BSU17340
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: hfq family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU17340
- UniProt: O31796
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (complex medium with amino acids, without glucose): 46 PubMed
Biological materials
- Mutant: GP22 (cat), available in the Jörg Stülke's lab
- Expression vector:
- lacZ fusion: pGP460 (in pAC7), available in Jörg Stülke's lab
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- FLAG-tag construct:
- GP1067 (spc, based on pGP1331), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications