Difference between revisions of "Hfq"
(→Original publications) |
|||
| (25 intermediate revisions by 4 users not shown) | |||
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || unknown | |style="background:#ABCDEF;" align="center"|'''Function''' || unknown | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU17340 hfq] | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 8 kDa, 8.698 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 8 kDa, 8.698 | ||
| Line 20: | Line 22: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[miaA]]'', ''[[ymzC]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[miaA]]'', ''[[ymzC]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU17340 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU17340 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU17340 DNA_with_flanks] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:ymaH_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:ymaH_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ymaH_1867373_1867594_1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:hfq_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU17340]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/> | ||
| − | + | = [[Categories]] containing this gene/protein = | |
| + | {{SubtiWiki category|[[RNA chaperones]]}} | ||
| + | |||
| + | = This gene is a member of the following [[regulons]] = | ||
=The gene= | =The gene= | ||
| Line 40: | Line 51: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU17340&redirect=T BSU17340] | ||
* '''DBTBS entry:''' no entry | * '''DBTBS entry:''' no entry | ||
| Line 46: | Line 58: | ||
=== Additional information=== | === Additional information=== | ||
| + | |||
| + | |||
| Line 70: | Line 84: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''[[SubtInteract|Interactions]]:''' |
| − | * '''Localization:''' | + | * '''[[Localization]]:''' |
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU17340&redirect=T BSU17340] | ||
| − | * '''Structure:''' [http://www.pdb.org/pdb/explore/explore.do?structureId=3HSB 3HSB] (complex with an RNA aptamer) | + | * '''Structure:''' [http://www.pdb.org/pdb/explore/explore.do?structureId=3HSB 3HSB] (complex with an RNA aptamer) {{PubMed|22053080}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/O31796 O31796] | * '''UniProt:''' [http://www.uniprot.org/uniprot/O31796 O31796] | ||
| Line 88: | Line 103: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[hfq]]'' {{PubMed|23457461}} |
| + | |||
| + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=ymaH_1867373_1867594_1 hfq] {{PubMed|22383849}} | ||
* '''[[Sigma factor]]:''' | * '''[[Sigma factor]]:''' | ||
| − | * '''Regulation:''' repressed by glucose (7.7-fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] | + | * '''Regulation:''' |
| + | ** repressed by glucose (7.7-fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] | ||
| + | ** expression (mRNA levels) is quite constant during growth in minimal medium {{PubMed|23457461}} | ||
| + | ** the Hfq protein amount increases upon transition to stationary phase {{PubMed|23457461}} | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| − | * '''Additional information:''' | + | * '''Additional information:''' |
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 46 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' GP22 (cat), available in the [[Stülke]] lab | + | * '''Mutant:''' GP22 (cat), available in the [[Jörg Stülke]]'s lab |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| − | * '''lacZ fusion:''' pGP460 (in [[pAC7]]), available in [[Stülke]] lab | + | * '''lacZ fusion:''' pGP460 (in [[pAC7]]), available in [[Jörg Stülke]]'s lab |
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * '''two-hybrid system:''' B. pertussis adenylate cyclase-based bacterial two hybrid system ([[BACTH]]), available in [[Stülke]] lab | + | * '''two-hybrid system:''' B. pertussis adenylate cyclase-based bacterial two hybrid system ([[BACTH]]), available in [[Jörg Stülke]]'s lab |
| + | |||
| + | * '''FLAG-tag construct:''' | ||
| + | ** GP1067 (spc, based on [[pGP1331]]), available in [[Jörg Stülke]]'s lab | ||
* '''Antibody:''' | * '''Antibody:''' | ||
| Line 118: | Line 142: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | <pubmed> 17395525 15009882 </pubmed> | + | <pubmed> 17395525 15009882 21760622 </pubmed> |
| + | |||
==Original publications== | ==Original publications== | ||
| − | <pubmed>12850135, 20445260 </pubmed> | + | <pubmed>12850135, 20445260 23457461 22965117,22053080 24932523 25150227 25915524</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Latest revision as of 15:41, 29 April 2015
- Description: RNA chaperone
| Gene name | hfq |
| Synonyms | ymaH |
| Essential | no |
| Product | RNA chaperone |
| Function | unknown |
| Gene expression levels in SubtiExpress: hfq | |
| MW, pI | 8 kDa, 8.698 |
| Gene length, protein length | 219 bp, 73 aa |
| Immediate neighbours | miaA, ymzC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 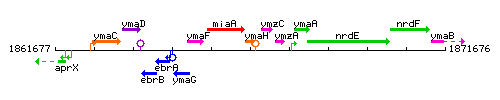
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17340
Phenotypes of a mutant
Database entries
- BsubCyc: BSU17340
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: hfq family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU17340
- UniProt: O31796
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (complex medium with amino acids, without glucose): 46 PubMed
Biological materials
- Mutant: GP22 (cat), available in the Jörg Stülke's lab
- Expression vector:
- lacZ fusion: pGP460 (in pAC7), available in Jörg Stülke's lab
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- FLAG-tag construct:
- GP1067 (spc, based on pGP1331), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications