Difference between revisions of "PtsI"
| Line 129: | Line 129: | ||
<pubmed>12850135 17218307, </pubmed> | <pubmed>12850135 17218307, </pubmed> | ||
| − | |||
| − | |||
| − | |||
Revision as of 14:04, 15 June 2009
- Description: Enzyme I, general (non sugar-specific) component of the PTS. Enzyme I transfers the phosphoryl group from phosphoenolpyruvate (PEP) to the phosphoryl carrier protein (HPr)
| Gene name | ptsI |
| Synonyms | |
| Essential | no |
| Product | phosphotransferase system (PTS) enzyme I |
| Function | PTS-dependent sugar transport |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 62,9 kDa, 4.59 |
| Gene length, protein length | 1710 bp, 570 amino acids |
| Immediate neighbours | ptsH, splA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 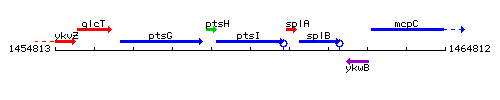
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU13910
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Phosphoenolpyruvate + protein L-histidine = pyruvate + protein N(pi)-phospho-L-histidine (according to Swiss-Prot) PEP-dependent autophosphorylation on His-189, transfer of the phosphoryl group to HPr (His-15)
- Protein family: PEP-utilizing enzyme family (according to Swiss-Prot) PEP-utilizing enzyme family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- HPr binding site (N-Terminal Domain)
- pyruvate binding site (C-Terminal Domain)
- pyrophosphate/phosphate carrier histidine (central Domain)
- Modification: transient autophosphorylation on His-189, in vivo also phosphorylated on Ser-34 or Ser-36 PubMed
- Cofactor(s): Magnesium
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot), Cytoplasm
Database entries
- Structure:
- Swiss prot entry: P08838
- KEGG entry: [3]
- E.C. number: 2.7.3.9 2.7.3.9]
Additional information
Expression and regulation
- Sigma factor: SigA PubMed
- Regulation: expression activated by glucose (4.3 fold) PubMed, induction by glucose (ptsG), constitutive (ptsH)
- Additional information:
Biological materials
- Mutant: GP864 (ermC), available in Stülke lab
- Expression vector: pAG3 (His-tag), available in Galinier lab
- lacZ fusion:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)