Difference between revisions of "ClpP"
| Line 125: | Line 125: | ||
=References= | =References= | ||
| − | <pubmed>9987115,11544224 14763982 9643546, </pubmed> | + | <pubmed>17380125,9643546,10809708,11807061,9987115,14679237,18689476,15317791,17586624,11684022,12923101,17560370,12598648,16899079,19226326,9890793,,9987115,11544224 14763982 9643546, </pubmed> |
# Petersohn et al. (2001) Global Analysis of the General Stress Response of ''Bacillus subtilis''. ''J Bacteriol.'' '''183:''' 5617-5631 [http://www.ncbi.nlm.nih.gov/pubmed/11544224 PubMed] | # Petersohn et al. (2001) Global Analysis of the General Stress Response of ''Bacillus subtilis''. ''J Bacteriol.'' '''183:''' 5617-5631 [http://www.ncbi.nlm.nih.gov/pubmed/11544224 PubMed] | ||
# Kock H, Gerth U, Hecker M. (2004) MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol Microbiol, 51:1087-1102. [http://www.ncbi.nlm.nih.gov/sites/entrez/14763982 PubMed] | # Kock H, Gerth U, Hecker M. (2004) MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol Microbiol, 51:1087-1102. [http://www.ncbi.nlm.nih.gov/sites/entrez/14763982 PubMed] | ||
Revision as of 09:22, 14 June 2009
- Description: ATP-dependent Clp protease proteolytic subunit (class III heat-shock protein)
| Gene name | clpP |
| Synonyms | yvdN |
| Essential | no |
| Product | ATP-dependent Clp protease proteolytic subunit |
| Function | protein degradation |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 21 kDa, 5.008 |
| Gene length, protein length | 591 bp, 197 aa |
| Immediate neighbours | trnQ-Arg, pgcM |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 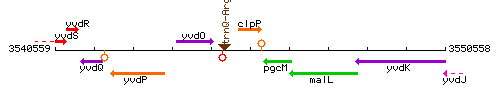
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU34540
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Hydrolysis of proteins to small peptides in the presence of ATP and magnesium (according to Swiss-Prot) endopeptidase/proteolysis
- Protein family: peptidase S14 family (according to Swiss-Prot) ClpP (IPR001907) InterPro, (PF00574) PFAM
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
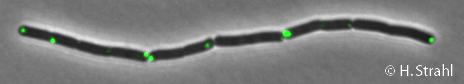
- Localization: cytoplasm (according to Swiss-Prot), cytoplasmic polar clusters, excluded from the nucleoid, induced clustering upon heatshock, colocalization with ClpX, ClpC and ClpE Pubmed
Database entries
- Structure: Two homologue structures resolved 1TYF, 1Y7O, structural model of B. subtilis ClpP available from hstrahl
- Swiss prot entry: P80244
- KEGG entry: [3]
- E.C. number: 3.4.21.92
Additional information
Expression and regulation
- Operon:
- Additional information:
Biological materials
- Mutant: clpP::spec and clpP::cat available from the Hamoen] Lab
- Expression vector:
- lacZ fusion:
- GFP fusion: C-terminal GFP fusions (both single copy and as 2th copy in amyE locus, also as CFP and YFP variants) available from the Hamoen] Lab
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Leendert Hamoen, Newcastle University, UK homepage
Your additional remarks
References
- Petersohn et al. (2001) Global Analysis of the General Stress Response of Bacillus subtilis. J Bacteriol. 183: 5617-5631 PubMed
- Kock H, Gerth U, Hecker M. (2004) MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol Microbiol, 51:1087-1102. PubMed
- Gerth, U., Krüger, E., Derré, I., Msadek, T. and Hecker, M. 1998. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the ClpP protease and the involvement of ClpP and ClpX in stress tolerance. Mol. Microbiol. 28: 787-802. PubMed
- Kirstein, J., Strahl, H., Molière, N., Hamoen, LW., Turgay K. (2008) Localization of general and regulatory proteolysis in Bacillus subtilis cells. Mol Microbiol. 70:682-94. Pubmed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed