Difference between revisions of "SucC"
Cadu Cunha (talk | contribs) (→Extended information on the protein) |
Cadu Cunha (talk | contribs) (→Additional information) |
||
| Line 87: | Line 87: | ||
=== Additional information=== | === Additional information=== | ||
| + | The enzyme is a dimer [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T36-44C8RWC-SH&_user=5731894&_coverDate=01%2F01%2F1985&_rdoc=28&_fmt=high&_orig=browse&_srch=doc-info(%23toc%234938%231985%23998209998%23270526%23FLP%23display%23Volume)&_cdi=4938&_sort=d&_docanchor=&_ct=40&_acct=C000043105&_version=1&_urlVersion=0&_userid=5731894&md5=f14f4734123ab1177d7217cab6c7ce7d FEBS Letters] | ||
=Expression and regulation= | =Expression and regulation= | ||
Revision as of 15:53, 10 June 2009
- Description: succinyl-CoA synthetase (beta subunit)
| Gene name | sucC |
| Synonyms | |
| Essential | no |
| Product | succinyl-CoA synthetase (beta subunit) |
| Function | TCA cycle |
| MW, pI | 41 kDa, 4.846 |
| Gene length, protein length | 1155 bp, 385 aa |
| Immediate neighbours | ylqH, sucD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 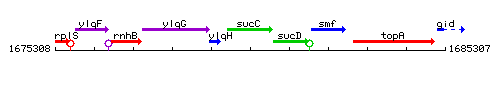
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU16090
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + succinate + CoA = ADP + phosphate + succinyl-CoA (according to Swiss-Prot)
- Protein family: ATP-grasp domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Reversible Michaelis-Menten FEBS Letters
- Domains:
- Modification: phosphorylation on Ser-220 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Inhibited by 2-oxoglutarate, ATP and NADH FEBS Letters
- GTP is not accept by the enzyme FEBS Letters
- Inhibited by 2-oxoglutarate, ATP and NADH FEBS Letters
- Interactions:
- Localization:
Database entries
- Structure: 1JKJ (E. coli)
- Swiss prot entry: P80886
- KEGG entry: [3]
- E.C. number: 6.2.1.5
Additional information
The enzyme is a dimer FEBS Letters
Expression and regulation
- Sigma factor:
- Regulatory mechanism: CcpA: transcription repression
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
- Blencke et al. (2003) Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab Eng. 5: 133-149 PubMed# Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6: 697-707 PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed