Difference between revisions of "Pyk"
Cadu Cunha (talk | contribs) (→Additional information) |
Cadu Cunha (talk | contribs) (→Additional information) |
||
| Line 91: | Line 91: | ||
=== Additional information=== | === Additional information=== | ||
| − | The enzyme is a tetramer with four active sites 3711058 | + | The enzyme is a tetramer with four active sites http://www.ncbi.nlm.nih.gov/pubmed/3711058 |
=Expression and regulation= | =Expression and regulation= | ||
Revision as of 13:19, 10 June 2009
- Description: pyruvate kinase, glycolytic enzyme
| Gene name | pyk |
| Synonyms | pykA |
| Essential | no |
| Product | pyruvate kinase |
| Function | catabolic enzyme in glycolysis |
| MW, pI | 61,9 kDa, 4.88 |
| Gene length, protein length | 1755 bp, 585 amino acids |
| Immediate neighbours | pfkA, ytzA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 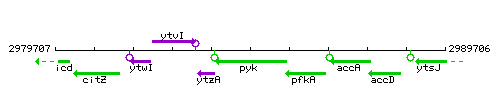
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU29180
Phenotypes of a mutant
Unable to grow with non-PTS carbohydrates (such as glucitol or glycerol) as single carbon source.
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ADP + phosphoenolpyruvate --> ATP + pyruvate
- The reaction is irreversible under physiological conditions
- Protein family: PEP-utilizing enzyme family (according to Swiss-Prot) pyruvate kinase family, (C-terminal section: PEP-utilizing enzyme family)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Allosteric Regulation PubMed
- Domains:
- Cofactor(s): Mg2+, K+
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm PubMed
Database entries
- Swiss prot entry: [3]
- KEGG entry: [4]
- E.C. number: 2.7.1.40
Additional information
The enzyme is a tetramer with four active sites http://www.ncbi.nlm.nih.gov/pubmed/3711058
Expression and regulation
- Sigma factor:
- Regulation: twofold induced by glucose PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP590 (cat), available in Stülke lab
- Expression vector:
Expression in E. coli, N-terminal His-tag: pGP1100 (in pWH844), available in Stülke lab
Expression in B. subtilis, native protein: pGP1411 (in pBQ200), available in Stülke lab
Expression in B. subtilis, N-terminal Strep-tag: pGP1409 (in pGP380), available in Stülke lab
Expression in B. subtilis, C-terminal Strep-tag: pGP1410 (in pGP382), available in Stülke lab
- lacZ fusion: see pfkA
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
- Eymann et al. (2007) Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis. Proteomics 7: 3509-3526. PubMed
- Lévine et al. (2006) Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes. Proteomics 6: 2157-2173 PubMed
- Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M., and Stülke, J. (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol Microbiol 41, 409-422.PubMed
- Jannière, L., Canceill, D., Suski, C., Kanga, S., Dalmais, B., Lestini, R., Monnier, A. F., Chapuis, J., Bolotin, A., Titok, M., Le Chatelier, E., and Ehrlich, S. D. (2007) Genetic evidence for a link between glycolysis and DNA replication. PLoS ONE 2, e447. PubMed
- Fry, B., Zhu, T., Domach, M. M., Koepsel, R. R., Phalakornkule, C., and Ataai, M. M. (2000) Characterization of growth and acid formation in a Bacillus subtilis pyruvate kinase mutant. Appl Env Microbiol 66: 4045-4049. PubMed
- Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6: 697-707 PubMed