Difference between revisions of "RpoA"
(→References) |
|||
| Line 119: | Line 119: | ||
=References= | =References= | ||
| + | |||
| + | <pubmed> 12642660, 14597697, 15659166, 16249335, 16740936</pubmed> | ||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 09:04, 30 May 2009
- Description: RNA polymerase alpha subunit
| Gene name | rpoA |
| Synonyms | |
| Essential | yes PubMed |
| Product | RNA polymerase alpha subunit |
| Function | transcription |
| MW, pI | 34 kDa, 4.593 |
| Gene length, protein length | 942 bp, 314 aa |
| Immediate neighbours | rpsK, rplQ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 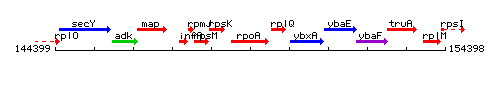
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1) (according to Swiss-Prot)
- Protein family: RNA polymerase alpha chain family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure: 1Z3E (C-terminal domain, complex with Spx)
- Swiss prot entry: P20429
- KEGG entry: BSU01430
- E.C. number: 2.7.7.6
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ying Zhang, Shunji Nakano, Soon-Yong Choi, Peter Zuber
Mutational analysis of the Bacillus subtilis RNA polymerase alpha C-terminal domain supports the interference model of Spx-dependent repression.
J Bacteriol: 2006, 188(12);4300-11
[PubMed:16740936]
[WorldCat.org]
[DOI]
(P p)
Kate J Newberry, Shunji Nakano, Peter Zuber, Richard G Brennan
Crystal structure of the Bacillus subtilis anti-alpha, global transcriptional regulator, Spx, in complex with the alpha C-terminal domain of RNA polymerase.
Proc Natl Acad Sci U S A: 2005, 102(44);15839-44
[PubMed:16249335]
[WorldCat.org]
[DOI]
(P p)
Shunji Nakano, Kyle N Erwin, Martina Ralle, Peter Zuber
Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx.
Mol Microbiol: 2005, 55(2);498-510
[PubMed:15659166]
[WorldCat.org]
[DOI]
(P p)
Shunji Nakano, Elke Küster-Schöck, Alan D Grossman, Peter Zuber
Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis.
Proc Natl Acad Sci U S A: 2003, 100(23);13603-8
[PubMed:14597697]
[WorldCat.org]
[DOI]
(P p)
Shunji Nakano, Michiko M Nakano, Ying Zhang, Montira Leelakriangsak, Peter Zuber
A regulatory protein that interferes with activator-stimulated transcription in bacteria.
Proc Natl Acad Sci U S A: 2003, 100(7);4233-8
[PubMed:12642660]
[WorldCat.org]
[DOI]
(P p)
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed