Difference between revisions of "RplJ"
| Line 110: | Line 110: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[rplJ]]-[[rplL]]'' {{PubMed|26101249}} |
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rplJ_120061_120561_1 rplJ] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rplJ_120061_120561_1 rplJ] {{PubMed|22383849}} | ||
| Line 118: | Line 118: | ||
* '''Regulation:''' | * '''Regulation:''' | ||
** [[RelA]] dependent downregulation (Class I) during stringent response {{PubMed|11948165}} | ** [[RelA]] dependent downregulation (Class I) during stringent response {{PubMed|11948165}} | ||
| + | ** termination/ antitermination ([[rplJ|L10]]-[[rplL|L12]]<sub>4</sub> complex) {{PubMed|26101249}} | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| + | ** [[rplJ|L10]]-[[rplL|L12]]<sub>4</sub> complex: transcription termination at excess of the complex (autorepression) {{PubMed|26101249}} | ||
* '''Additional information:''' | * '''Additional information:''' | ||
Revision as of 09:22, 23 July 2015
- Description: ribosomal protein
| Gene name | rplJ |
| Synonyms | |
| Essential | yes PubMed |
| Product | ribosomal protein L10 (BL5) |
| Function | translation |
| Gene expression levels in SubtiExpress: rplJ | |
| Interactions involving this protein in SubtInteract: RplJ | |
| MW, pI | 17 kDa, 5.489 |
| Gene length, protein length | 498 bp, 166 aa |
| Immediate neighbours | rplA, rplL |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 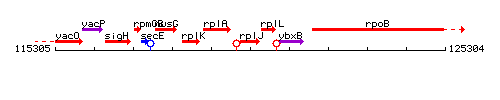
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed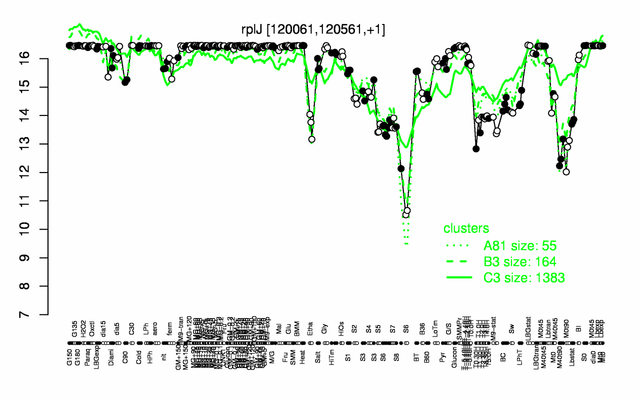
| |
Contents
Categories containing this gene/protein
translation, essential genes, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01040
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU01040
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein L10P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU01040
- Structure:
- UniProt: P42923
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- belongs to the 100 most abundant proteins PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 1318 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 7379 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 5113 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 2638 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 2690 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Helen Yakhnin, Alexander V Yakhnin, Paul Babitzke
Ribosomal protein L10(L12)4 autoregulates expression of the Bacillus subtilis rplJL operon by a transcription attenuation mechanism.
Nucleic Acids Res: 2015, 43(14);7032-43
[PubMed:26101249]
[WorldCat.org]
[DOI]
(I p)
Genki Akanuma, Hideaki Nanamiya, Yousuke Natori, Koichi Yano, Shota Suzuki, Shuya Omata, Morio Ishizuka, Yasuhiko Sekine, Fujio Kawamura
Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation.
J Bacteriol: 2012, 194(22);6282-91
[PubMed:23002217]
[WorldCat.org]
[DOI]
(I p)
Matthew A Lauber, William E Running, James P Reilly
B. subtilis ribosomal proteins: structural homology and post-translational modifications.
J Proteome Res: 2009, 8(9);4193-206
[PubMed:19653700]
[WorldCat.org]
[DOI]
(P p)
James R Iben, David E Draper
Specific interactions of the L10(L12)4 ribosomal protein complex with mRNA, rRNA, and L11.
Biochemistry: 2008, 47(9);2721-31
[PubMed:18247578]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Annette Dreisbach, Dirk Albrecht, Jörg Bernhardt, Dörte Becher, Sandy Gentner, Le Thi Tam, Knut Büttner, Gerrit Buurman, Christian Scharf, Simone Venz, Uwe Völker, Michael Hecker
A comprehensive proteome map of growing Bacillus subtilis cells.
Proteomics: 2004, 4(10);2849-76
[PubMed:15378759]
[WorldCat.org]
[DOI]
(P p)