Difference between revisions of "KinB"
(→References) |
|||
| Line 153: | Line 153: | ||
=References= | =References= | ||
| − | <pubmed>8576055,16166384,9299348,8497199,10094672,9426145,11069677,12618455,11902725,12618455,7592498, 21097618 19101565 23378509 23599347 </pubmed> | + | <pubmed>8576055,16166384,9299348,8497199,10094672,9426145,11069677,12618455,11902725,12618455,7592498, 21097618 19101565 23378509 23599347 26152584</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:05, 13 July 2015
- Description: two-component sensor kinase, phosphorylates Spo0F, part of the phosphorelay, senses changes in respiratory activity
| Gene name | kinB |
| Synonyms | |
| Essential | no |
| Product | two-component sensor kinase |
| Function | initiation of sporulation |
| Gene expression levels in SubtiExpress: kinB | |
| Interactions involving this protein in SubtInteract: KinB | |
| Function and regulation of this protein in SubtiPathways: kinB | |
| MW, pI | 47 kDa, 6.682 |
| Gene length, protein length | 1287 bp, 429 aa |
| Immediate neighbours | patB, kapB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 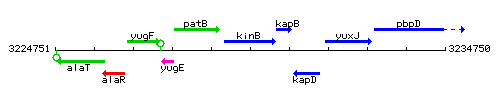
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
protein modification, transcription factors and their control, phosphorelay, membrane proteins, phosphoproteins
This gene is a member of the following regulons
CodY regulon, stringent response
The gene
Basic information
- Locus tag: BSU31450
Phenotypes of a mutant
Database entries
- BsubCyc: BSU31450
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: six transmembrane segments, C-terminal histidine phosphotransferase domain
- Modification: autophosphorylation on a His residue
- Cofactor(s):
- Effectors of protein activity:
- activity is triggered at low respiratory activity, this depends on a functional interaction with the respiration apparatus PubMed
- Localization: cell membrane (integral membrane protein) PubMed
Database entries
- BsubCyc: BSU31450
- UniProt: Q08430
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- repressed during growth in the presence of branched chain amino acids (CodY) PubMed
- induced upon addition of decoyinine (positive stringent response) PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Roberto R Grau, Paula de Oña, Maritta Kunert, Cecilia Leñini, Ramses Gallegos-Monterrosa, Eisha Mhatre, Darío Vileta, Verónica Donato, Theresa Hölscher, Wilhelm Boland, Oscar P Kuipers, Ákos T Kovács
A Duo of Potassium-Responsive Histidine Kinases Govern the Multicellular Destiny of Bacillus subtilis.
mBio: 2015, 6(4);e00581
[PubMed:26152584]
[WorldCat.org]
[DOI]
(I e)
Ilana Kolodkin-Gal, Alexander K W Elsholz, Christine Muth, Peter R Girguis, Roberto Kolter, Richard Losick
Respiration control of multicellularity in Bacillus subtilis by a complex of the cytochrome chain with a membrane-embedded histidine kinase.
Genes Dev: 2013, 27(8);887-99
[PubMed:23599347]
[WorldCat.org]
[DOI]
(I p)
Shigeo Tojo, Kazutake Hirooka, Yasutaro Fujita
Expression of kinA and kinB of Bacillus subtilis, necessary for sporulation initiation, is under positive stringent transcription control.
J Bacteriol: 2013, 195(8);1656-65
[PubMed:23378509]
[WorldCat.org]
[DOI]
(I p)
Anna L McLoon, Ilana Kolodkin-Gal, Shmuel M Rubinstein, Roberto Kolter, Richard Losick
Spatial regulation of histidine kinases governing biofilm formation in Bacillus subtilis.
J Bacteriol: 2011, 193(3);679-85
[PubMed:21097618]
[WorldCat.org]
[DOI]
(I p)
Matthew J Bick, Valerie Lamour, Kanagalaghatta R Rajashankar, Yuliya Gordiyenko, Carol V Robinson, Seth A Darst
How to switch off a histidine kinase: crystal structure of Geobacillus stearothermophilus KinB with the inhibitor Sda.
J Mol Biol: 2009, 386(1);163-77
[PubMed:19101565]
[WorldCat.org]
[DOI]
(I p)
Masaya Fujita, Richard Losick
Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A.
Genes Dev: 2005, 19(18);2236-44
[PubMed:16166384]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Yoshiko Nakaura, Robert P Shivers, Hirotake Yamaguchi, Richard Losick, Yasutaro Fujita, Abraham L Sonenshein
Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis.
J Bacteriol: 2003, 185(6);1911-22
[PubMed:12618455]
[WorldCat.org]
[DOI]
(P p)
M Jiang, W Shao, M Perego, J A Hoch
Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis.
Mol Microbiol: 2000, 38(3);535-42
[PubMed:11069677]
[WorldCat.org]
[DOI]
(P p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)
V Dartois, T Djavakhishvili, J A Hoch
KapB is a lipoprotein required for KinB signal transduction and activation of the phosphorelay to sporulation in Bacillus subtilis.
Mol Microbiol: 1997, 26(5);1097-108
[PubMed:9426145]
[WorldCat.org]
[DOI]
(P p)
Y L Tzeng, J A Hoch
Molecular recognition in signal transduction: the interaction surfaces of the Spo0F response regulator with its cognate phosphorelay proteins revealed by alanine scanning mutagenesis.
J Mol Biol: 1997, 272(2);200-12
[PubMed:9299348]
[WorldCat.org]
[DOI]
(P p)
V Dartois, J Liu, J A Hoch
Alterations in the flow of one-carbon units affect KinB-dependent sporulation in Bacillus subtilis.
Mol Microbiol: 1997, 25(1);39-51
[PubMed:11902725]
[WorldCat.org]
[DOI]
(P p)
V Dartois, T Djavakhishvili, J A Hoch
Identification of a membrane protein involved in activation of the KinB pathway to sporulation in Bacillus subtilis.
J Bacteriol: 1996, 178(4);1178-86
[PubMed:8576055]
[WorldCat.org]
[DOI]
(P p)
M A Strauch
Delineation of AbrB-binding sites on the Bacillus subtilis spo0H, kinB, ftsAZ, and pbpE promoters and use of a derived homology to identify a previously unsuspected binding site in the bsuB1 methylase promote.
J Bacteriol: 1995, 177(23);6999-7002
[PubMed:7592498]
[WorldCat.org]
[DOI]
(P p)
K A Trach, J A Hoch
Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway.
Mol Microbiol: 1993, 8(1);69-79
[PubMed:8497199]
[WorldCat.org]
[DOI]
(P p)