Difference between revisions of "Sda"
| Line 47: | Line 47: | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| + | {{SubtiWiki regulon|[[DnaA regulon]]}}, | ||
{{SubtiWiki regulon|[[LexA regulon]]}} | {{SubtiWiki regulon|[[LexA regulon]]}} | ||
| Line 92: | Line 93: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
** [[KinB]]-[[Sda]] {{PubMed|19465772}} | ** [[KinB]]-[[Sda]] {{PubMed|19465772}} | ||
| − | ** | + | ** [[KinA]]-[[Sda]] {{PubMed|15023339}} |
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| Line 108: | Line 109: | ||
=== Additional information=== | === Additional information=== | ||
| − | + | * [[Sda]] is rapidly degraded by the [[ClpP|ClpP/X]] system because of some uncharged residues at its C-terminus {{PubMed|16796683}} | |
| − | Sda is rapidly | ||
=Expression and regulation= | =Expression and regulation= | ||
| Line 123: | Line 123: | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| − | ** | + | ** [[DnaA]]: transcription activation under replication stress {{PubMed|17932079}} |
** [[LexA]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/16267290 PubMed] | ** [[LexA]]: transcription repression [http://www.ncbi.nlm.nih.gov/sites/entrez/16267290 PubMed] | ||
| Line 155: | Line 155: | ||
<pubmed> 22933559</pubmed> | <pubmed> 22933559</pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>11207367, 16267290, 19465772, 15023339 16796683 19684115 17932079 17350039 19101565 20525796</pubmed> | + | <pubmed>11207367, 16267290, 19465772, 15023339 16796683 19684115 17932079 17350039 19101565 20525796 26020636</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:20, 1 June 2015
- Description: developmental checkpoint protein, inhibits the autokinase activity of KinA and KinB, control of the phosphorelay
| Gene name | sda |
| Synonyms | |
| Essential | no |
| Product | developmental checkpoint protein |
| Function | mediates a developmental checkpoint coupling initiation of sporulation (phosphorylation of Spo0A) to the function of replication initiation proteins |
| Gene expression levels in SubtiExpress: sda | |
| Interactions involving this protein in SubtInteract: Sda | |
| Function and regulation of this protein in SubtiPathways: sda | |
| MW, pI | 6 kDa, 5.605 |
| Gene length, protein length | 156 bp, 52 aa |
| Immediate neighbours | yloV, yqeF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 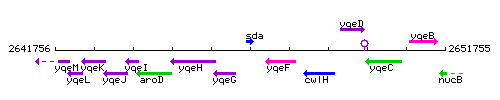
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU25690
Phenotypes of a mutant
- sporulates at a higher frequency
- will sporulate in the presence of replication stress
Database entries
- BsubCyc: BSU25690
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Sda is a checkpoint protein that is upregulated in response to replication stress PubMed. Sda inhibits the autokinase activity of KinA (and likely KinB as well). This in its turn results in reduced levels of Spo0A~P. Thus, replication stressed cells will not initiate sporulation.
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU25690
- UniProt: Q7WY62
- KEGG entry: [3]
- E.C. number:
Additional information
- Sda is rapidly degraded by the ClpP/X system because of some uncharged residues at its C-terminus PubMed
Expression and regulation
- Regulatory mechanism:
- Additional information:
- Sda is rapidly turned over by the ClpP/X system because of some uncharged residues at its C-terminus PubMed.
- An antisense RNA is predicted for sda PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications