Difference between revisions of "FtsZ"
(→Database entries) |
|||
| Line 118: | Line 118: | ||
* '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU15290&redirect=T BSU15290] | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU15290&redirect=T BSU15290] | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=2VAM 2VAM] | + | * '''Structure:''' |
| + | ** [http://www.rcsb.org/pdb/explore.do?structureId=2VAM 2VAM] | ||
| + | ** [http://www.rcsb.org/pdb/explore.do?structureId=2RHL 2RHL] (dimer with GDP) | ||
| + | ** [http://www.rcsb.org/pdb/explore/explore.do?structureId=4u39 4U39] ([[FtsZ]]-[[MciZ]] complex) {{PubMed|25848052}} | ||
* '''UniProt:''' [http://www.uniprot.org/uniprot/P17865 P17865] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P17865 P17865] | ||
Revision as of 07:02, 9 April 2015
- Description: cell-division initiation protein (septum formation)
| Gene name | ftsZ |
| Synonyms | ts-1 |
| Essential | yes PubMed |
| Product | cell-division initiation protein (septum formation) |
| Function | formation of Z-ring |
| Gene expression levels in SubtiExpress: ftsZ | |
| Interactions involving this protein in SubtInteract: FtsZ | |
| MW, pI | 40 kDa, 4.814 |
| Gene length, protein length | 1146 bp, 382 aa |
| Immediate neighbours | ftsA, bpr |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 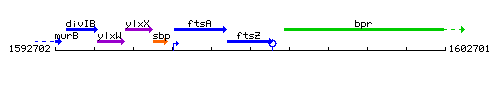
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell division, essential genes, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15290
Phenotypes of a mutant
Database entries
- BsubCyc: BSU15290
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ftsZ family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Z ring formation is inhibited upon binding of MciZ to FtsZ
- bundling of FtsZ protofilaments into strikingly long and regular tubular structures reminiscent of eukaryotic microtubules requires the prior formation of large ring polymers of SepF PubMed
- interaction with UgtP inhibits FtsZ filament formation PubMed
- FtsZ polymerization is inhibited by interaction with MinC PubMed
- Z ring formation requires PdhA in a pyruvate-dependent manner PubMed
- Localization:
- septal at the cell membrane PubMed
- septal localization partially depends on the proton motive force PubMed
- Noc and the Min system ensure the efficient utilization of the division site at midcell in by ensuring Z ring placement PubMed
- FtsZ is anchored to the cell membrane by either FtsA or SepF PubMed
Database entries
- BsubCyc: BSU15290
- UniProt: P17865
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- strains:
- GP1372 (Pxyl ftsZ aphA3) disA::tet cdaS::ermC for xylose inducible expression of ftsZ, available in Jörg Stülke's lab
- Expression vector:
- GP2009: expression of ftsZ-Strep under control of the ftsZ promoter (based on pGP1389), available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody: available in the Jeff Errington lab
Labs working on this gene/protein
- Imrich Barak, Slovak Academy of Science, Bratislava, Slovakia homepage
- Leendert Hamoen, CBCB, Newcastle University, UK
Your additional remarks
References
Reviews
FtsZ as antibacterial drug target
Other original Publications