Difference between revisions of "DesK"
(→Reviews) |
(→Original publications) |
||
| Line 154: | Line 154: | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>10094672,14734164,12399512,20507988 , 19233289 11285232 19805278 15090506, 12207704 20705470 23356219 24574048 24522108 </pubmed> | + | <pubmed>10094672,14734164,12399512,20507988 , 19233289 11285232 19805278 15090506, 12207704 20705470 23356219 24574048 24522108 25406381 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 07:02, 24 November 2014
- Description: two-component sensor kinase, regulation of cold shock expression of des
| Gene name | desK |
| Synonyms | yocF |
| Essential | no |
| Product | two-component sensor kinase |
| Function | regulation of cold shock expression of des |
| Gene expression levels in SubtiExpress: desK | |
| Interactions involving this protein in SubtInteract: DesK | |
| Metabolic function and regulation of this protein in SubtiPathways: desK | |
| MW, pI | 42 kDa, 9.428 |
| Gene length, protein length | 1110 bp, 370 aa |
| Immediate neighbours | des, desR |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 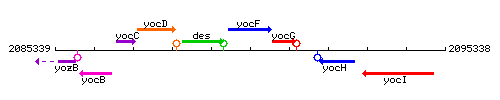
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed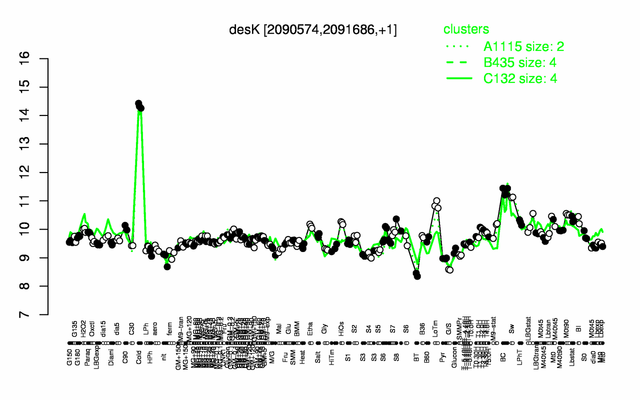
| |
Contents
Categories containing this gene/protein
lipid metabolism/ other, protein modification, transcription factors and their control, cold stress proteins, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU19190
Phenotypes of a mutant
Database entries
- BsubCyc: BSU19190
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: autophosphorylation, phosphorylation of DesR
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- 5 transmembrane helices
- cytoplasmatic C-terminal trail
- Modification: autophosphorylation on a His residue
- Cofactor(s):
- Effectors of protein activity: unsaturated fatty acids are negative effectors of the system
- Localization: membrane (transmembrane segments)
Database entries
- BsubCyc: BSU19190
- Structure: 3EHF
- UniProt: O34757
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- induced by cold shock (12-fold) PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Diego de Mendoza, Universidad Nacional de Rosario, Argentine homepage
- Mohamed Marahiel, Marburg University, Germany homepage
Your additional remarks
References
Reviews
Diego de Mendoza
Temperature sensing by membranes.
Annu Rev Microbiol: 2014, 68;101-16
[PubMed:24819366]
[WorldCat.org]
[DOI]
(I p)
Richard C Stewart
Protein histidine kinases: assembly of active sites and their regulation in signaling pathways.
Curr Opin Microbiol: 2010, 13(2);133-41
[PubMed:20117042]
[WorldCat.org]
[DOI]
(I p)
Pablo S Aguilar, Diego de Mendoza
Control of fatty acid desaturation: a mechanism conserved from bacteria to humans.
Mol Microbiol: 2006, 62(6);1507-14
[PubMed:17087771]
[WorldCat.org]
[DOI]
(P p)
Original publications
Felipe Trajtenberg, Daniela Albanesi, Natalia Ruétalo, Horacio Botti, Ariel E Mechaly, Marcos Nieves, Pablo S Aguilar, Larisa Cybulski, Nicole Larrieux, Diego de Mendoza, Alejandro Buschiazzo
Allosteric activation of bacterial response regulators: the role of the cognate histidine kinase beyond phosphorylation.
mBio: 2014, 5(6);e02105
[PubMed:25406381]
[WorldCat.org]
[DOI]
(I e)
Lucía Porrini, Larisa E Cybulski, Silvia G Altabe, María C Mansilla, Diego de Mendoza
Cerulenin inhibits unsaturated fatty acids synthesis in Bacillus subtilis by modifying the input signal of DesK thermosensor.
Microbiologyopen: 2014, 3(2);213-24
[PubMed:24574048]
[WorldCat.org]
[DOI]
(I p)
María Eugenia Inda, Michel Vandenbranden, Ariel Fernández, Diego de Mendoza, Jean-Marie Ruysschaert, Larisa Estefanía Cybulski
A lipid-mediated conformational switch modulates the thermosensing activity of DesK.
Proc Natl Acad Sci U S A: 2014, 111(9);3579-84
[PubMed:24522108]
[WorldCat.org]
[DOI]
(I p)
Mariana Martín, Diego de Mendoza
Regulation of Bacillus subtilis DesK thermosensor by lipids.
Biochem J: 2013, 451(2);269-75
[PubMed:23356219]
[WorldCat.org]
[DOI]
(I p)
Larisa E Cybulski, Mariana Martín, María C Mansilla, Ariel Fernández, Diego de Mendoza
Membrane thickness cue for cold sensing in a bacterium.
Curr Biol: 2010, 20(17);1539-44
[PubMed:20705470]
[WorldCat.org]
[DOI]
(I p)
Felipe Trajtenberg, Martin Graña, Natalia Ruétalo, Horacio Botti, Alejandro Buschiazzo
Structural and enzymatic insights into the ATP binding and autophosphorylation mechanism of a sensor histidine kinase.
J Biol Chem: 2010, 285(32);24892-903
[PubMed:20507988]
[WorldCat.org]
[DOI]
(I p)
Daniela Albanesi, Mariana Martín, Felipe Trajtenberg, María C Mansilla, Ahmed Haouz, Pedro M Alzari, Diego de Mendoza, Alejandro Buschiazzo
Structural plasticity and catalysis regulation of a thermosensor histidine kinase.
Proc Natl Acad Sci U S A: 2009, 106(38);16185-90
[PubMed:19805278]
[WorldCat.org]
[DOI]
(I p)
Mariana Martín, Daniela Albanesi, Pedro M Alzari, Diego de Mendoza
Functional in vitro assembly of the integral membrane bacterial thermosensor DesK.
Protein Expr Purif: 2009, 66(1);39-45
[PubMed:19233289]
[WorldCat.org]
[DOI]
(I p)
Daniela Albanesi, María Cecilia Mansilla, Diego de Mendoza
The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator.
J Bacteriol: 2004, 186(9);2655-63
[PubMed:15090506]
[WorldCat.org]
[DOI]
(P p)
Karen Hunger, Carsten L Beckering, Mohamed A Marahiel
Genetic evidence for the temperature-sensing ability of the membrane domain of the Bacillus subtilis histidine kinase DesK.
FEMS Microbiol Lett: 2004, 230(1);41-6
[PubMed:14734164]
[WorldCat.org]
[DOI]
(P p)
Carsten L Beckering, Leif Steil, Michael H W Weber, Uwe Völker, Mohamed A Marahiel
Genomewide transcriptional analysis of the cold shock response in Bacillus subtilis.
J Bacteriol: 2002, 184(22);6395-402
[PubMed:12399512]
[WorldCat.org]
[DOI]
(P p)
Larisa E Cybulski, Daniela Albanesi, María C Mansilla, Silvia Altabe, Pablo S Aguilar, Diego de Mendoza
Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase.
Mol Microbiol: 2002, 45(5);1379-88
[PubMed:12207704]
[WorldCat.org]
[DOI]
(P p)
P S Aguilar, A M Hernandez-Arriaga, L E Cybulski, A C Erazo, D de Mendoza
Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis.
EMBO J: 2001, 20(7);1681-91
[PubMed:11285232]
[WorldCat.org]
[DOI]
(P p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)