Difference between revisions of "SivA"
| (30 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' inhibitor of [[KinA]] autophosphorylation, and subsequently of entry into [[sporulation]] <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || inhibitor of [[KinA]] autophosphorylation |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || control of entry into [[sporulation]] via the [[phosphorelay]] |
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU37800 sivA] | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 16 kDa, 7.175 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 16 kDa, 7.175 | ||
| Line 20: | Line 22: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[rocG]]'', ''[[spsL]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[rocG]]'', ''[[spsL]]'' | ||
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''[http:// | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU37800 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU37800 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU37800 DNA_with_flanks] |
| − | |||
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:yweA_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:yweA_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=yweA_3882191_3882655_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:yweA_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU37800]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/> | ||
| − | + | = [[Categories]] containing this gene/protein = | |
| + | {{SubtiWiki category|[[phosphorelay]]}} | ||
| + | |||
| + | = This gene is a member of the following [[regulons]] = | ||
| + | {{SubtiWiki regulon|[[AbrB regulon]]}} | ||
=The gene= | =The gene= | ||
| Line 36: | Line 47: | ||
=== Basic information === | === Basic information === | ||
| − | * ''' | + | * '''Locus tag:''' BSU37800 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | + | * the'' [[sivA]] [[bslA]]'' double mutant exhibits a more severe loss of repellency of the biofilm surface as compared to the ''[[bslA]]'' mutant {{PubMed|22571672}} | |
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU37800&redirect=T BSU37800] | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/yweA.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/yweA.html] | ||
| Line 47: | Line 59: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
=The protein= | =The protein= | ||
| Line 54: | Line 65: | ||
* '''Catalyzed reaction/ biological activity:''' | * '''Catalyzed reaction/ biological activity:''' | ||
| + | ** inhibits [[KinA]] autophophorylation {{PubMed|23335417}} | ||
* '''Protein family:''' | * '''Protein family:''' | ||
| − | * '''Paralogous protein(s):''' | + | * '''Paralogous protein(s):''' [[BslA]] |
=== Extended information on the protein === | === Extended information on the protein === | ||
| Line 71: | Line 83: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''[[SubtInteract|Interactions]]:''' |
| − | * '''Localization:''' extracellular (signal peptide) [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed] | + | * '''[[Localization]]:''' |
| + | ** membrane (according to Swiss-Prot) | ||
| + | ** extracellular (signal peptide) [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed] | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU37800&redirect=T BSU37800] | ||
* '''Structure:''' | * '''Structure:''' | ||
| − | * ''' | + | * '''UniProt:''' [http://www.uniprot.org/uniprot/P39632 P39632] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU37800] |
* '''E.C. number:''' | * '''E.C. number:''' | ||
| Line 88: | Line 103: | ||
=Expression and regulation= | =Expression and regulation= | ||
| + | * '''Operon:''' ''sivA'' {{PubMed|12823818}} | ||
| − | * ''' | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=yweA_3882191_3882655_-1 sivA] {{PubMed|22383849}} |
* '''[[Sigma factor]]:''' | * '''[[Sigma factor]]:''' | ||
| Line 96: | Line 112: | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| + | ** [[AbrB]]: transcription repression {{PubMed|20817675}} | ||
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
| Line 118: | Line 135: | ||
=References= | =References= | ||
| + | <pubmed>18957862, 12823818 22571672 23335417 20817675</pubmed> | ||
| − | + | [[Category:Protein-coding genes]] | |
| − | |||
Latest revision as of 15:06, 2 April 2014
- Description: inhibitor of KinA autophosphorylation, and subsequently of entry into sporulation
| Gene name | yweA |
| Synonyms | ipa-74d |
| Essential | no |
| Product | inhibitor of KinA autophosphorylation |
| Function | control of entry into sporulation via the phosphorelay |
| Gene expression levels in SubtiExpress: sivA | |
| MW, pI | 16 kDa, 7.175 |
| Gene length, protein length | 462 bp, 154 aa |
| Immediate neighbours | rocG, spsL |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 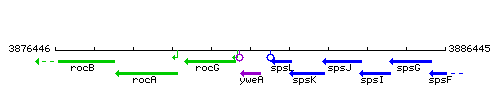
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed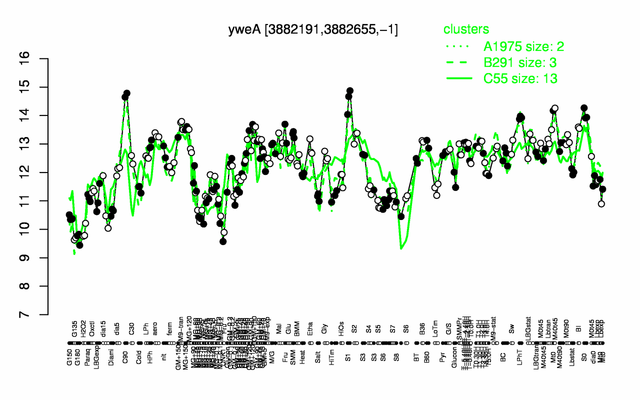
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU37800
Phenotypes of a mutant
- the sivA bslA double mutant exhibits a more severe loss of repellency of the biofilm surface as compared to the bslA mutant PubMed
Database entries
- BsubCyc: BSU37800
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Protein family:
- Paralogous protein(s): BslA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- membrane (according to Swiss-Prot)
- extracellular (signal peptide) PubMed
Database entries
- BsubCyc: BSU37800
- Structure:
- UniProt: P39632
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: sivA PubMed
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Sharon Garti-Levi, Ashlee Eswara, Yoav Smith, Masaya Fujita, Sigal Ben-Yehuda
Novel modulators controlling entry into sporulation in Bacillus subtilis.
J Bacteriol: 2013, 195(7);1475-83
[PubMed:23335417]
[WorldCat.org]
[DOI]
(I p)
Kazuo Kobayashi, Megumi Iwano
BslA(YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms.
Mol Microbiol: 2012, 85(1);51-66
[PubMed:22571672]
[WorldCat.org]
[DOI]
(I p)
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Ken-ichi Yoshida, Hirotake Yamaguchi, Masaki Kinehara, Yo-hei Ohki, Yoshiko Nakaura, Yasutaro Fujita
Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box.
Mol Microbiol: 2003, 49(1);157-65
[PubMed:12823818]
[WorldCat.org]
[DOI]
(P p)