Difference between revisions of "XynC"
| (39 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | * '''Description:''' | + | * '''Description:''' endo-xylanase, preference for methylglucurono-xylan <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || endo-xylanase |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || xylan degradation |
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU18150 xynC] | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 47 kDa, 9.078 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 47 kDa, 9.078 | ||
| Line 20: | Line 22: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ynfE]]'', ''[[xynD]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[ynfE]]'', ''[[xynD]]'' | ||
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''[http:// | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU18150 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU18150 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU18150 DNA_with_flanks] |
| − | |||
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:ynfF_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:ynfF_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=xynC_1942714_1943982_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:xynC_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU18150]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/> | ||
| − | + | = [[Categories]] containing this gene/protein = | |
| + | {{SubtiWiki category|[[utilization of specific carbon sources]]}} | ||
| + | |||
| + | = This gene is a member of the following [[regulons]] = | ||
| + | {{SubtiWiki regulon|[[AbrB regulon]]}} | ||
=The gene= | =The gene= | ||
| Line 36: | Line 47: | ||
=== Basic information === | === Basic information === | ||
| − | * ''' | + | * '''Locus tag:''' BSU18150 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU18150&redirect=T BSU18150] | ||
* '''DBTBS entry:''' no entry | * '''DBTBS entry:''' no entry | ||
| Line 47: | Line 59: | ||
=== Additional information=== | === Additional information=== | ||
| + | |||
| + | |||
| Line 53: | Line 67: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' Endohydrolysis of (1->4)-beta-D-xylosyl links in some glucuronoarabinoxylans (according to Swiss-Prot) |
| − | * '''Protein family:''' | + | * '''Protein family:''' glycosyl hydrolase 30 family (according to Swiss-Prot) |
* '''Paralogous protein(s):''' | * '''Paralogous protein(s):''' | ||
| Line 71: | Line 85: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''[[SubtInteract|Interactions]]:''' |
| − | * '''Localization:''' extracellular (signal peptide) [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed] | + | * '''[[Localization]]:''' |
| + | ** extracellular (signal peptide) [http://www.ncbi.nlm.nih.gov/pubmed/18957862 PubMed] | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU18150&redirect=T BSU18150] | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=3GTN 3GTN] {{PubMed|19407387,21256135}} |
| − | * ''' | + | * '''UniProt:''' [http://www.uniprot.org/uniprot/Q45070 Q45070] |
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU18150] |
| − | * '''E.C. number:''' | + | * '''E.C. number:''' [http://www.expasy.org/enzyme/3.2.1.136 3.2.1.136] |
=== Additional information=== | === Additional information=== | ||
=Expression and regulation= | =Expression and regulation= | ||
| + | * '''Operon:''' ''[[xynD]]-[[xynC]]'' {{PubMed|20817675}} | ||
| − | * ''' | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=xynC_1942714_1943982_-1 xynC] {{PubMed|22383849}} |
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
* '''Regulation:''' | * '''Regulation:''' | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| + | ** [[AbrB]]: transcription repression {{PubMed|20817675}} | ||
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
| Line 118: | Line 136: | ||
=References= | =References= | ||
| + | ==Reviews== | ||
| + | <pubmed>20735481 </pubmed> | ||
| + | ==Original publications== | ||
| + | <pubmed>19407387, 18957862, 17028274 20817675,21256135 24271172 </pubmed> | ||
| − | + | [[Category:Protein-coding genes]] | |
| − | |||
Latest revision as of 13:51, 2 April 2014
- Description: endo-xylanase, preference for methylglucurono-xylan
| Gene name | xynC |
| Synonyms | ynfF |
| Essential | no |
| Product | endo-xylanase |
| Function | xylan degradation |
| Gene expression levels in SubtiExpress: xynC | |
| MW, pI | 47 kDa, 9.078 |
| Gene length, protein length | 1266 bp, 422 aa |
| Immediate neighbours | ynfE, xynD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 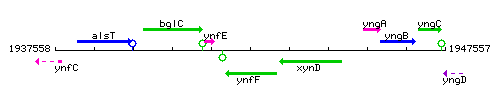
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU18150
Phenotypes of a mutant
Database entries
- BsubCyc: BSU18150
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Endohydrolysis of (1->4)-beta-D-xylosyl links in some glucuronoarabinoxylans (according to Swiss-Prot)
- Protein family: glycosyl hydrolase 30 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- extracellular (signal peptide) PubMed
Database entries
- BsubCyc: BSU18150
- UniProt: Q45070
- KEGG entry: [2]
- E.C. number: 3.2.1.136
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Mun Su Rhee, Lusha Wei, Neha Sawhney, John D Rice, Franz J St John, Jason C Hurlbert, James F Preston
Engineering the xylan utilization system in Bacillus subtilis for production of acidic Xylooligosaccharides.
Appl Environ Microbiol: 2014, 80(3);917-27
[PubMed:24271172]
[WorldCat.org]
[DOI]
(I p)
Franz J St John, Jason C Hurlbert, John D Rice, James F Preston, Edwin Pozharski
Ligand bound structures of a glycosyl hydrolase family 30 glucuronoxylan xylanohydrolase.
J Mol Biol: 2011, 407(1);92-109
[PubMed:21256135]
[WorldCat.org]
[DOI]
(I p)
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Franz J St John, David K Godwin, James F Preston, Edwin Pozharski, Jason C Hurlbert
Crystallization and crystallographic analysis of Bacillus subtilis xylanase C.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2009, 65(Pt 5);499-503
[PubMed:19407387]
[WorldCat.org]
[DOI]
(I p)
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Franz J St John, John D Rice, James F Preston
Characterization of XynC from Bacillus subtilis subsp. subtilis strain 168 and analysis of its role in depolymerization of glucuronoxylan.
J Bacteriol: 2006, 188(24);8617-26
[PubMed:17028274]
[WorldCat.org]
[DOI]
(P p)