Difference between revisions of "CheD"
| Line 57: | Line 57: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU16460&redirect=T BSU16460] | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/fla-che.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/fla-che.html] | ||
| Line 99: | Line 100: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU16460&redirect=T BSU16460] | ||
* '''Structure:''' | * '''Structure:''' | ||
Revision as of 13:44, 2 April 2014
- Description: chemoreceptor deaminase, required for methylation of methyl-accepting chemotaxis proteins by CheR, enhances phosphatase activity of CheC, required for full McpC activity
| Gene name | cheD |
| Synonyms | ylxK |
| Essential | no |
| Product | protein deaminase, CheC activity modulator |
| Function | motility and chemotaxis |
| Gene expression levels in SubtiExpress: cheD | |
| Interactions involving this protein in SubtInteract: CheD | |
| MW, pI | 17 kDa, 9.347 |
| Gene length, protein length | 498 bp, 166 aa |
| Immediate neighbours | cheC, sigD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 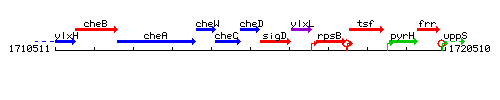
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
protein modification, motility and chemotaxis
This gene is a member of the following regulons
CodY regulon, SigD regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU16460
Phenotypes of a mutant
Database entries
- BsubCyc: BSU16460
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: cheD family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU16460
- Structure:
- UniProt: P40404
- KEGG entry: [3]
- E.C. number: 3.5.1.44
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
- in minimal medium, CheD is present with 1,200 +/- 250 molecules per cell PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
___
____