Difference between revisions of "GlnA"
| Line 45: | Line 45: | ||
{{SubtiWiki category|[[transcription factors and their control]]}}, | {{SubtiWiki category|[[transcription factors and their control]]}}, | ||
{{SubtiWiki category|[[trigger enzyme]]}}, | {{SubtiWiki category|[[trigger enzyme]]}}, | ||
| − | {{SubtiWiki category|[[phosphoproteins]]}} | + | {{SubtiWiki category|[[phosphoproteins]]}}, |
| + | [[most abundant proteins]] | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| Line 83: | Line 84: | ||
* '''Kinetic information:''' K(M) for: Glu: 27 mM, ATP: 2.4 mM, ammonium: 0.18 mM; v(max): 3.7 µmol/min/mg | * '''Kinetic information:''' K(M) for: Glu: 27 mM, ATP: 2.4 mM, ammonium: 0.18 mM; v(max): 3.7 µmol/min/mg | ||
| − | * '''Domains:''' glutamate binding flap (aa 300 ... 306: protects unstable intermediates from abberant hydrolysis) | + | * '''[[Domains]]:''' glutamate binding flap (aa 300 ... 306: protects unstable intermediates from abberant hydrolysis) |
* '''Modification:''' | * '''Modification:''' | ||
| Line 89: | Line 90: | ||
** ''in vitro'' phosphorylated by [[PrkC]] on Thr-26, Thr-147, Ser-207, and Thr-286 {{PubMed|20389117}} | ** ''in vitro'' phosphorylated by [[PrkC]] on Thr-26, Thr-147, Ser-207, and Thr-286 {{PubMed|20389117}} | ||
| − | * ''' | + | * '''[[Cofactors]]:''' Mg(2+) |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 137: | Line 138: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** belongs to the 100 [[most abundant proteins]] {{PubMed|15378759}} | ||
=Biological materials = | =Biological materials = | ||
| Line 165: | Line 167: | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>19233925, 20389117,8799114,18195355, 11719184, 12139611, 2573733, 8636055, 19233925, 16493705, 16885465, 6141156 2906311 20656908 16055443 18331450 16547045 8093698 21435182 23535029 24158439 </pubmed> | + | <pubmed>19233925, 20389117,8799114,18195355, 11719184, 12139611, 2573733, 8636055, 19233925, 16493705, 16885465, 6141156 2906311 20656908 16055443 18331450 16547045 8093698 21435182 23535029 24158439 15378759</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:01, 5 March 2014
- Description: trigger enzyme: glutamine synthetase and effector of TnrA and GlnR
| Gene name | glnA |
| Synonyms | |
| Essential | no |
| Product | trigger enzyme: glutamine synthetase |
| Function | glutamine biosynthesis, control of TnrA and GlnR activity |
| Gene expression levels in SubtiExpress: glnA | |
| Interactions involving this protein in SubtInteract: GlnA | |
| Metabolic function and regulation of this protein in SubtiPathways: glnA | |
| MW, pI | 50 kDa, 4.874 |
| Gene length, protein length | 1332 bp, 444 aa |
| Immediate neighbours | glnR, ynxB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 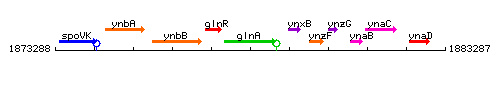
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, glutamate metabolism, transcription factors and their control, trigger enzyme, phosphoproteins, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17460
Phenotypes of a mutant
auxotrophic for glutamine
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-glutamate + NH3 = ADP + phosphate + L-glutamine (according to Swiss-Prot)
- Protein family: glutamine synthetase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: K(M) for: Glu: 27 mM, ATP: 2.4 mM, ammonium: 0.18 mM; v(max): 3.7 µmol/min/mg
- Domains: glutamate binding flap (aa 300 ... 306: protects unstable intermediates from abberant hydrolysis)
- Modification:
- Cofactors: Mg(2+)
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- 4LNN (apo-GS) PubMed
- 3QAJ (complex with ATP)
- A general discussion of GS structure
- UniProt: P12425
- KEGG entry: [3]
- E.C. number: 6.3.1.2
Additional information
GlnA is a homooligomer of 12 subunits
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant: GP247 (glnA::cat), available in Stülke lab
- Expression vector:
- expression/ purification from E. coli, with N-terminal Strep-tag (in pGP172): pGP174, available in Stülke lab
- pGP177 (N-terminal Strep-tag, purification from B. subtilis, for SPINE, in pBQ200), available in Jörg Stülke's lab
- GFP fusion:
- two-hybrid system:
- Antibody: available in Karl Forchhammer lab
Labs working on this gene/protein
Susan Fisher, Boston, USA homepage
Your additional remarks
References
Reviews
Katrin Gunka, Fabian M Commichau
Control of glutamate homeostasis in Bacillus subtilis: a complex interplay between ammonium assimilation, glutamate biosynthesis and degradation.
Mol Microbiol: 2012, 85(2);213-24
[PubMed:22625175]
[WorldCat.org]
[DOI]
(I p)
Fabian M Commichau, Jörg Stülke
Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression.
Mol Microbiol: 2008, 67(4);692-702
[PubMed:18086213]
[WorldCat.org]
[DOI]
(P p)
S H Fisher
Regulation of nitrogen metabolism in Bacillus subtilis: vive la différence!
Mol Microbiol: 1999, 32(2);223-32
[PubMed:10231480]
[WorldCat.org]
[DOI]
(P p)
Original publications