Difference between revisions of "MutS"
| Line 80: | Line 80: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 100: | Line 100: | ||
* '''Structure:''' | * '''Structure:''' | ||
| + | ** [http://www.pdb.org/pdb/explore/explore.do?structureId=1ng9 1NG9] (from ''E. coli'', 39% identity) {{PubMed|12554674}} | ||
* '''UniProt:''' [http://www.uniprot.org/uniprot/P49849 P49849] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P49849 P49849] | ||
| Line 149: | Line 150: | ||
<pubmed> 22933559 </pubmed> | <pubmed> 22933559 </pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>8760914, 15375129,16479537 20525796 20453097 23228104 21958350 23882084 23998896 24062730 </pubmed> | + | <pubmed>8760914, 15375129,16479537 20525796 20453097 23228104 21958350 23882084 23998896 24062730 12554674 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:24, 27 February 2014
- Description: DNA mismatch repair (mismatch recognition protein)
| Gene name | mutS |
| Synonyms | |
| Essential | no |
| Product | mismatch recognition protein |
| Function | DNA repair |
| Gene expression levels in SubtiExpress: mutS | |
| Interactions involving this protein in SubtInteract: MutS | |
| MW, pI | 97 kDa, 5.189 |
| Gene length, protein length | 2574 bp, 858 aa |
| Immediate neighbours | cotE, mutL |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 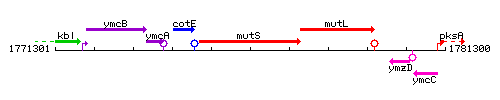
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed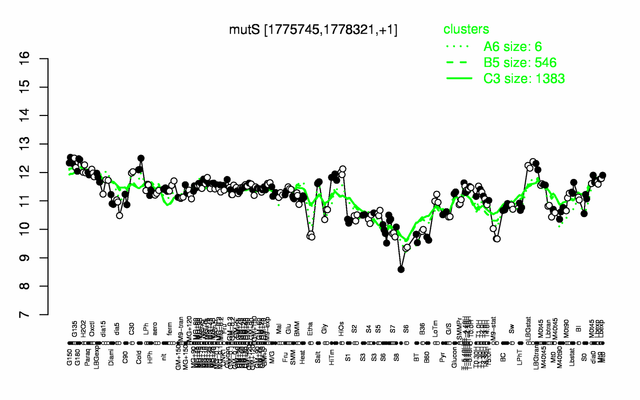
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17040
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- MutS recognizes mismatches in the DNA and recruits MutL to the site of mismatch recognition
- Protein family: DNA mismatch repair mutS family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- UniProt: P49849
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- An antisense RNA is predicted for mutS PubMed
- intracellular concentration: about 80 dimers (100 nM) PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications