Difference between revisions of "Crh"
(→Biological materials) |
|||
| Line 136: | Line 136: | ||
=Biological materials = | =Biological materials = | ||
| − | * '''Mutant:''' GP860 (aphA3), QB7097 (spc), available in [[Stülke]] lab | + | * '''Mutant:''' GP860 (aphA3) {{PubMed|21992469}}, QB7097 (spc), available in [[Stülke]] lab |
* '''Expression vector:''' | * '''Expression vector:''' | ||
| Line 149: | Line 149: | ||
* '''FLAG-tag construct: | * '''FLAG-tag construct: | ||
| − | ** GP69 (spc, based on [[pGP1331]]), available in the [[Boris Görke]] and [[Stülke]] labs | + | ** GP69 (spc, based on [[pGP1331]]) {{PubMed|21992469}}, available in the [[Boris Görke]] and [[Stülke]] labs |
** pGP1200 ''crh''(Ser46Ala) (spc, based on [[pGP1331]]), available in the [[Stülke]] lab | ** pGP1200 ''crh''(Ser46Ala) (spc, based on [[pGP1331]]), available in the [[Stülke]] lab | ||
| − | |||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
Revision as of 17:45, 16 January 2014
- Description: "Carbon-flux regulating HPr", formerly known as "Catabolite repression HPr-like protein", control of flux through the harmful methylglyoxal pathway, minor cofactor of the CcpA transcription factor
| Gene name | crh |
| Synonyms | yvcM |
| Essential | no |
| Product | carbon-flux regulating HPr |
| Function | control of carbon flux |
| Gene expression levels in SubtiExpress: crh | |
| Interactions involving this protein in SubtInteract: Crh | |
| Metabolic function and regulation of this protein in SubtiPathways: crh | |
| MW, pI | 9,2 kDa, 4.70 |
| Gene length, protein length | 255 bp, 85 amino acids |
| Immediate neighbours | yvcN, yvcL |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 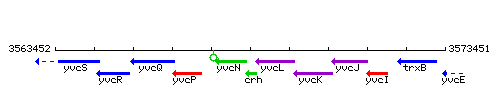
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transcription factors and their control, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU34740
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: HPr family
- Paralogous protein(s): HPr
Extended information on the protein
- Kinetic information:
- Domains: HPr domain (1–85)
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: O06976
- KEGG entry: [2]
Additional information
Crh does not possess the phosphorylation site used for PTS phosphotransfer (His-15 in PtsH), it can only be phosphorylated on Ser-46
Expression and regulation
- Regulation: very weak stimuation of expression by citrate and succinate PubMed
- Regulatory mechanism:
- Additional information: Crh is weakly expressed. This results in part from a poorly conserved ribosomal binding site of the mRNA. PubMed
Biological materials
- Expression vector:
- lacZ fusion: see yvcI
- GFP fusion:
- two-hybrid system: crh, crh(Ser46Asp), crh(Ser46Ala) B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Görke lab
- FLAG-tag construct:
Labs working on this gene/protein
Boris Görke, University of Göttingen, Germany Homepage
Anne Galinier, University of Marseille, France
Wolfgang Hillen, Erlangen University, Germany Homepage
Richard Brennan, Houston, Texas, USA Homepage
Your additional remarks
References
Jens J Landmann, Susanne Werner, Wolfgang Hillen, Jörg Stülke, Boris Görke
Carbon source control of the phosphorylation state of the Bacillus subtilis carbon-flux regulator Crh in vivo.
FEMS Microbiol Lett: 2012, 327(1);47-53
[PubMed:22092971]
[WorldCat.org]
[DOI]
(I p)
Jens J Landmann, Ricarda A Busse, Jan-Hendrik Latz, Kalpana D Singh, Jörg Stülke, Boris Görke
Crh, the paralogue of the phosphocarrier protein HPr, controls the methylglyoxal bypass of glycolysis in Bacillus subtilis.
Mol Microbiol: 2011, 82(3);770-87
[PubMed:21992469]
[WorldCat.org]
[DOI]
(I p)
Carole Gardiennet, Antoine Loquet, Manuel Etzkorn, Henrike Heise, Marc Baldus, Anja Böckmann
Structural constraints for the Crh protein from solid-state NMR experiments.
J Biomol NMR: 2008, 40(4);239-50
[PubMed:18320329]
[WorldCat.org]
[DOI]
(P p)
Antoine Loquet, Benjamin Bardiaux, Carole Gardiennet, Christophe Blanchet, Marc Baldus, Michael Nilges, Thérèse Malliavin, Anja Böckmann
3D structure determination of the Crh protein from highly ambiguous solid-state NMR restraints.
J Am Chem Soc: 2008, 130(11);3579-89
[PubMed:18284240]
[WorldCat.org]
[DOI]
(I p)
Frédérique Pompeo, Jennifer Luciano, Anne Galinier
Interaction of GapA with HPr and its homologue, Crh: Novel levels of regulation of a key step of glycolysis in Bacillus subtilis?
J Bacteriol: 2007, 189(3);1154-7
[PubMed:17142398]
[WorldCat.org]
[DOI]
(P p)
Vincent Chaptal, Laurent Larivière, Virginie Gueguen-Chaignon, Anne Galinier, Sylvie Nessler, Solange Moréra
X-ray structure of a domain-swapped dimer of Ser46-phosphorylated Crh from Bacillus subtilis.
Proteins: 2006, 63(1);249-51
[PubMed:16411239]
[WorldCat.org]
[DOI]
(I p)
Maria A Schumacher, Gerald Seidel, Wolfgang Hillen, Richard G Brennan
Phosphoprotein Crh-Ser46-P displays altered binding to CcpA to effect carbon catabolite regulation.
J Biol Chem: 2006, 281(10);6793-800
[PubMed:16316990]
[WorldCat.org]
[DOI]
(P p)
Boris Görke, Elodie Foulquier, Anne Galinier
YvcK of Bacillus subtilis is required for a normal cell shape and for growth on Krebs cycle intermediates and substrates of the pentose phosphate pathway.
Microbiology (Reading): 2005, 151(Pt 11);3777-3791
[PubMed:16272399]
[WorldCat.org]
[DOI]
(P p)
Boris Görke, Laetitia Fraysse, Anne Galinier
Drastic differences in Crh and HPr synthesis levels reflect their different impacts on catabolite repression in Bacillus subtilis.
J Bacteriol: 2004, 186(10);2992-5
[PubMed:15126459]
[WorldCat.org]
[DOI]
(P p)
Michel Juy, François Penin, Adrien Favier, Anne Galinier, Roland Montserret, Richard Haser, Josef Deutscher, Anja Böckmann
Dimerization of Crh by reversible 3D domain swapping induces structural adjustments to its monomeric homologue Hpr.
J Mol Biol: 2003, 332(4);767-76
[PubMed:12972249]
[WorldCat.org]
[DOI]
(P p)
Jessica B Warner, Juke S Lolkema
A Crh-specific function in carbon catabolite repression in Bacillus subtilis.
FEMS Microbiol Lett: 2003, 220(2);277-80
[PubMed:12670692]
[WorldCat.org]
[DOI]
(P p)
Jean-Pierre Lavergne, Jean-Michel Jault, Anne Galinier
Insights into the functioning of Bacillus subtilis HPr kinase/phosphatase: affinity for its protein substrates and role of cations and phosphate.
Biochemistry: 2002, 41(20);6218-25
[PubMed:12009882]
[WorldCat.org]
[DOI]
(P p)
Adrien Favier, Bernhard Brutscher, Martin Blackledge, Anne Galinier, Josef Deutscher, François Penin, Dominique Marion
Solution structure and dynamics of Crh, the Bacillus subtilis catabolite repression HPr.
J Mol Biol: 2002, 317(1);131-44
[PubMed:11916384]
[WorldCat.org]
[DOI]
(P p)
E Darbon, A Galinier, D Le Coq, J Deutscher
Phosphotransfer functions mutated Bacillus subtilis HPr-like protein Crh carrying a histidine in the active site.
J Mol Microbiol Biotechnol: 2001, 3(3);439-44
[PubMed:11361076]
[WorldCat.org]
(P p)
F Penin, A Favier, R Montserret, B Brutscher, J Deutscher, D Marion, D Galinier
Evidence for a dimerisation state of the Bacillus subtilis catabolite repression HPr-like protein, Crh.
J Mol Microbiol Biotechnol: 2001, 3(3);429-32
[PubMed:11361074]
[WorldCat.org]
(P p)
Isabelle Martin-Verstraete, Anne Galinier, Emmanuelle Darbon, Yves Quentin, Marie-Claude Kilhoffer, Véronique Charrier, Jacques Haiech, Georges Rapoport, Josef Deutscher
The Q15H mutation enables Crh, a Bacillus subtilis HPr-like protein, to carry out some regulatory HPr functions, but does not make it an effective phosphocarrier for sugar transport.
Microbiology (Reading): 1999, 145 ( Pt 11);3195-3204
[PubMed:10589728]
[WorldCat.org]
[DOI]
(P p)
I Martin-Verstraete, J Deutscher, A Galinier
Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signalling pathway for the Bacillus subtilis levanase operon.
J Bacteriol: 1999, 181(9);2966-9
[PubMed:10217795]
[WorldCat.org]
[DOI]
(P p)
A Galinier, J Deutscher, I Martin-Verstraete
Phosphorylation of either crh or HPr mediates binding of CcpA to the bacillus subtilis xyn cre and catabolite repression of the xyn operon.
J Mol Biol: 1999, 286(2);307-14
[PubMed:9973552]
[WorldCat.org]
[DOI]
(P p)
A Galinier, J Haiech, M C Kilhoffer, M Jaquinod, J Stülke, J Deutscher, I Martin-Verstraete
The Bacillus subtilis crh gene encodes a HPr-like protein involved in carbon catabolite repression.
Proc Natl Acad Sci U S A: 1997, 94(16);8439-44
[PubMed:9237995]
[WorldCat.org]
[DOI]
(P p)