Difference between revisions of "RplQ"
| Line 90: | Line 90: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| + | ** [[Map]]-[[RplQ]] {{PubMed|23770820}} | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| Line 139: | Line 140: | ||
=References= | =References= | ||
| − | <pubmed>8635744,23002217, 19653700 </pubmed> | + | <pubmed>8635744,23002217, 19653700 23770820</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 12:28, 20 August 2013
- Description: ribosomal protein
| Gene name | rplQ |
| Synonyms | |
| Essential | yes PubMed |
| Product | ribosomal protein L17 (BL15) |
| Function | translation |
| Gene expression levels in SubtiExpress: rplQ | |
| Interactions involving this protein in SubtInteract: RplQ | |
| MW, pI | 13 kDa, 10.265 |
| Gene length, protein length | 360 bp, 120 aa |
| Immediate neighbours | rpoA, ybxA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed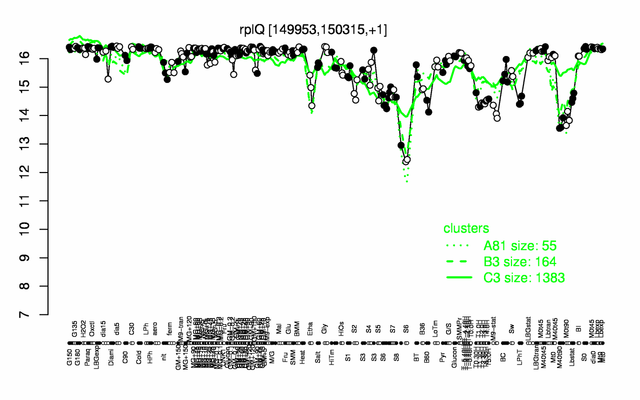
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01440
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein L17P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P20277
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Arzu Sandikci, Felix Gloge, Michael Martinez, Matthias P Mayer, Rebecca Wade, Bernd Bukau, Günter Kramer
Dynamic enzyme docking to the ribosome coordinates N-terminal processing with polypeptide folding.
Nat Struct Mol Biol: 2013, 20(7);843-50
[PubMed:23770820]
[WorldCat.org]
[DOI]
(I p)
Genki Akanuma, Hideaki Nanamiya, Yousuke Natori, Koichi Yano, Shota Suzuki, Shuya Omata, Morio Ishizuka, Yasuhiko Sekine, Fujio Kawamura
Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation.
J Bacteriol: 2012, 194(22);6282-91
[PubMed:23002217]
[WorldCat.org]
[DOI]
(I p)
Matthew A Lauber, William E Running, James P Reilly
B. subtilis ribosomal proteins: structural homology and post-translational modifications.
J Proteome Res: 2009, 8(9);4193-206
[PubMed:19653700]
[WorldCat.org]
[DOI]
(P p)
J W Suh, S A Boylan, S H Oh, C W Price
Genetic and transcriptional organization of the Bacillus subtilis spc-alpha region.
Gene: 1996, 169(1);17-23
[PubMed:8635744]
[WorldCat.org]
[DOI]
(P p)