Difference between revisions of "UgtP"
(→Original Publications) |
|||
| Line 154: | Line 154: | ||
==Original Publications== | ==Original Publications== | ||
| − | + | <pubmed>9244290,18820022,17662947,9720862 22362028 15640167 23935518 22931116</pubmed> | |
| − | <pubmed>9244290,18820022,17662947,9720862 22362028 15640167 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:48, 13 August 2013
- Description: UDP-glucose diacylglycerol glucosyltransferase, growth-rate dependent inhibitor of cell division
| Gene name | ugtP |
| Synonyms | ypfP |
| Essential | no |
| Product | UDP-glucose diacylglycerol glucosyltransferase |
| Function | synthesis of glycolipids and anchoring of lipoteichoic acid, inhibition of FtsZ assembly |
| Gene expression levels in SubtiExpress: ugtP | |
| Interactions involving this protein in SubtInteract: UgtP | |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis | |
| MW, pI | 43 kDa, 8.398 |
| Gene length, protein length | 1146 bp, 382 aa |
| Immediate neighbours | metA, cspD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 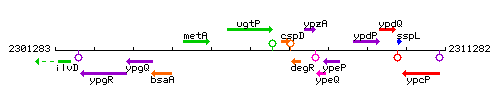
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed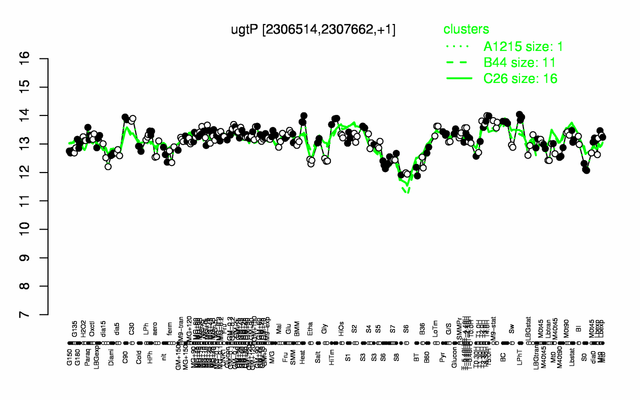
| |
Contents
Categories containing this gene/protein
cell division, lipid metabolism/ other, cell envelope stress proteins (controlled by SigM, V, W, X, Y), membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU21920
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- UDP-glucose + 1,2-diacylglycerol = UDP + 1,2-diacyl-3-(O-beta-D-glucopyranosyl)-sn-glycerol (according to Swiss-Prot)
- the interaction with FtsZ results in inhibition of cell division and an increase of cell size PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- membrane-bound protein, self-assembles into tightly wound spirals in vitro PubMed
- under nutrient rich conditions (increased concentration of UDP-Glc): throughout the cell, concentrated at the cell poles and/or the cytokinetic ring, interaction with FtsZ PubMed
- under nutrient poor conditions: forms punctate foci (oligomers), no interaction with FtsZ PubMed
Database entries
- Structure:
- UniProt: P54166
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original Publications
Norbert S Hill, Paul J Buske, Yue Shi, Petra Anne Levin
A moonlighting enzyme links Escherichia coli cell size with central metabolism.
PLoS Genet: 2013, 9(7);e1003663
[PubMed:23935518]
[WorldCat.org]
[DOI]
(I p)
An-Chun Chien, Shannon Kian Gharabiklou Zareh, Yan Mei Wang, Petra Anne Levin
Changes in the oligomerization potential of the division inhibitor UgtP co-ordinate Bacillus subtilis cell size with nutrient availability.
Mol Microbiol: 2012, 86(3);594-610
[PubMed:22931116]
[WorldCat.org]
[DOI]
(I p)
Satoshi Matsuoka, Minako Chiba, Yu Tanimura, Michihiro Hashimoto, Hiroshi Hara, Kouji Matsumoto
Abnormal morphology of Bacillus subtilis ugtP mutant cells lacking glucolipids.
Genes Genet Syst: 2011, 86(5);295-304
[PubMed:22362028]
[WorldCat.org]
[DOI]
(I p)
Letal I Salzberg, John D Helmann
Phenotypic and transcriptomic characterization of Bacillus subtilis mutants with grossly altered membrane composition.
J Bacteriol: 2008, 190(23);7797-807
[PubMed:18820022]
[WorldCat.org]
[DOI]
(I p)
Richard B Weart, Amy H Lee, An-Chun Chien, Daniel P Haeusser, Norbert S Hill, Petra Anne Levin
A metabolic sensor governing cell size in bacteria.
Cell: 2007, 130(2);335-47
[PubMed:17662947]
[WorldCat.org]
[DOI]
(P p)
Vladimir Lazarevic, Blazenka Soldo, Noël Médico, Harold Pooley, Sierd Bron, Dimitri Karamata
Bacillus subtilis alpha-phosphoglucomutase is required for normal cell morphology and biofilm formation.
Appl Environ Microbiol: 2005, 71(1);39-45
[PubMed:15640167]
[WorldCat.org]
[DOI]
(P p)
P Jorasch, F P Wolter, U Zähringer, E Heinz
A UDP glucosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1,2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products.
Mol Microbiol: 1998, 29(2);419-30
[PubMed:9720862]
[WorldCat.org]
[DOI]
(P p)
K D Price, S Roels, R Losick
A Bacillus subtilis gene encoding a protein similar to nucleotide sugar transferases influences cell shape and viability.
J Bacteriol: 1997, 179(15);4959-61
[PubMed:9244290]
[WorldCat.org]
[DOI]
(P p)