Difference between revisions of "PrsW"
(→References) |
|||
| Line 141: | Line 141: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | + | <pubmed>22688815 23479438,22381678</pubmed> | |
| − | + | == Original publications == | |
| − | ==Original | + | <pubmed>16816000,17020587,19889088 , 23155385</pubmed> |
| − | |||
| − | <pubmed>16816000,17020587,19889088 , </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:52, 31 July 2013
- Description: protease, cleaves RsiW in the presence of antimicrobial peptides
| Gene name | prsW |
| Synonyms | ypdC |
| Essential | no |
| Product | protease |
| Function | control of SigW activity |
| Gene expression levels in SubtiExpress: prsW | |
| Interactions involving this protein in SubtInteract: PrsW | |
| MW, pI | 24 kDa, 8.085 |
| Gene length, protein length | 654 bp, 218 aa |
| Immediate neighbours | sleB, ypdA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 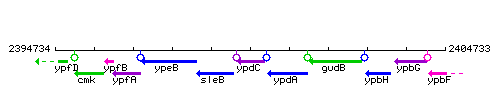
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed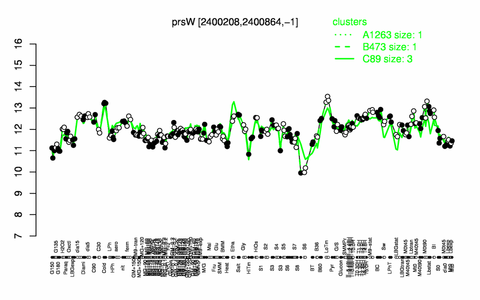
| |
Contents
Categories containing this gene/protein
proteolysis, sigma factors and their control, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU22940
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: protease prsW family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: membrane PubMed
Database entries
- Structure:
- UniProt: P50738
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Thomas Wiegert, University of Bayreuth, Germany Homepage
Your additional remarks
References
Reviews
Noël Molière, Kürşad Turgay
General and regulatory proteolysis in Bacillus subtilis.
Subcell Biochem: 2013, 66;73-103
[PubMed:23479438]
[WorldCat.org]
[DOI]
(P p)
Ross E Dalbey, Peng Wang, Jan Maarten van Dijl
Membrane proteases in the bacterial protein secretion and quality control pathway.
Microbiol Mol Biol Rev: 2012, 76(2);311-30
[PubMed:22688815]
[WorldCat.org]
[DOI]
(I p)
Theresa D Ho, Craig D Ellermeier
Extra cytoplasmic function σ factor activation.
Curr Opin Microbiol: 2012, 15(2);182-8
[PubMed:22381678]
[WorldCat.org]
[DOI]
(I p)
Original publications