Difference between revisions of "SpeD"
(→References) |
|||
| Line 143: | Line 143: | ||
=References= | =References= | ||
| − | + | <pubmed>10844697,15720552 ,9723923 21815947 </pubmed> | |
| − | |||
| − | |||
| − | |||
| − | <pubmed>10844697,15720552 ,9723923 | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:35, 13 July 2013
- Description: S-adenosylmethionine decarboxylase
| Gene name | speD |
| Synonyms | ytcF |
| Essential | no |
| Product | S-adenosylmethionine decarboxylase |
| Function | spermidine, polyamine biosynthesis |
| Gene expression levels in SubtiExpress: speD | |
| Metabolic function and regulation of this protein in SubtiPathways: Cys, Met & Sulfate assimilation, Central C-metabolism | |
| MW, pI | 13 kDa, 4.768 |
| Gene length, protein length | 384 bp, 128 aa |
| Immediate neighbours | ytcG, gapB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 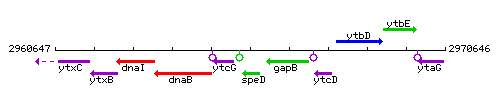
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed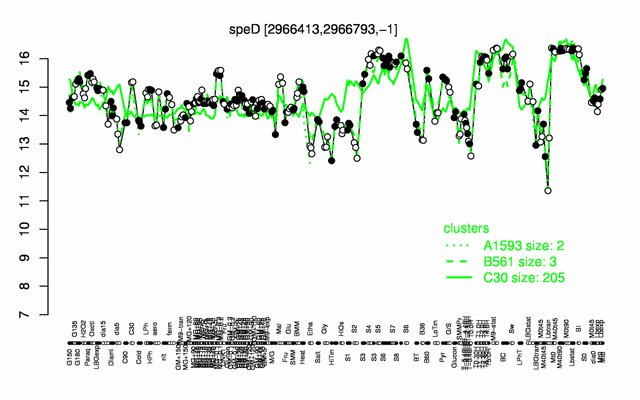
| |
Contents
Categories containing this gene/protein
miscellaneous metabolic pathways
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29010
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: S-adenosyl-L-methionine = (5-deoxy-5-adenosyl)(3-aminopropyl)-methylsulfonium salt + CO2 (according to Swiss-Prot)
- Protein family: Type 1 subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1VR7 (from Thermotoga maritima, 47% identity, 72% similarity)
- UniProt: O34426
- KEGG entry: [3]
- E.C. number: 4.1.1.50
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Martin Lehnik-Habrink, Marc Schaffer, Ulrike Mäder, Christine Diethmaier, Christina Herzberg, Jörg Stülke
RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y.
Mol Microbiol: 2011, 81(6);1459-73
[PubMed:21815947]
[WorldCat.org]
[DOI]
(I p)
Pascale Servant, Dominique Le Coq, Stéphane Aymerich
CcpN (YqzB), a novel regulator for CcpA-independent catabolite repression of Bacillus subtilis gluconeogenic genes.
Mol Microbiol: 2005, 55(5);1435-51
[PubMed:15720552]
[WorldCat.org]
[DOI]
(P p)
A Sekowska, J Y Coppée, J P Le Caer, I Martin-Verstraete, A Danchin
S-adenosylmethionine decarboxylase of Bacillus subtilis is closely related to archaebacterial counterparts.
Mol Microbiol: 2000, 36(5);1135-47
[PubMed:10844697]
[WorldCat.org]
[DOI]
(P p)
A Sekowska, P Bertin, A Danchin
Characterization of polyamine synthesis pathway in Bacillus subtilis 168.
Mol Microbiol: 1998, 29(3);851-8
[PubMed:9723923]
[WorldCat.org]
[DOI]
(P p)