Difference between revisions of "PdhB"
| Line 26: | Line 26: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[pdhA]]'', ''[[pdhC]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[pdhA]]'', ''[[pdhC]]'' | ||
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU14590 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU14590 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU14590 | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU14590 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU14590 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU14590 DNA_with_flanks] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:pdhB_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:pdhB_context.gif]] | ||
Revision as of 10:06, 14 May 2013
- Description: pyruvate dehydrogenase (E1 beta subunit)
| Gene name | pdhB |
| Synonyms | |
| Essential | no |
| Product | pyruvate dehydrogenase (E1 beta subunit) |
| Function | links glycolysis and TCA cycle |
| Gene expression levels in SubtiExpress: pdhB | |
| Interactions involving this protein in SubtInteract: PdhB | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 35 kDa, 4.547 |
| Gene length, protein length | 975 bp, 325 aa |
| Immediate neighbours | pdhA, pdhC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 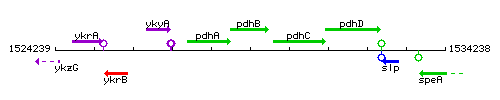
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
carbon core metabolism, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14590
Phenotypes of a mutant
- defects in sporulation and unable to grow on glucose as single carbon source PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Pyruvate + [dihydrolipoyllysine-residue acetyltransferase] lipoyllysine = [dihydrolipoyllysine-residue acetyltransferase] S-acetyldihydrolipoyllysine + CO2 (according to Swiss-Prot)
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Michaelis-Menten PubMed
- Domains:
- Modification: phosphorylation on (Ser-302 OR Ser-306) PubMed
- Cofactor(s):
- Effectors of protein activity:
- Inhibited thiamine 2-thiothiazolone diphosphate and NADH PubMed
- Low sensibility to NADPH
- Localization: membrane associated PubMed
Database entries
- Structure: 1W88 (E1 in complex with subunit binding domain of E2, Geobacillus stearothermophilus)
- UniProt: P21882
- KEGG entry: [3]
- E.C. number: 1.2.4.1
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- stringent response: due to presence of guanine at +1 position of the transcript PubMed
- Additional information:
Biological materials
- Mutant: GP459 (spc), available in Stülke lab
- Expression vector:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Arthur Aronson, Purdue University, West Lafayette, USA homepage
Your additional remarks
References
Reviews
Original publications