Difference between revisions of "CcpA"
(→References) |
|||
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || mediates carbon catabolite repression (CCR) | |style="background:#ABCDEF;" align="center"|'''Function''' || mediates carbon catabolite repression (CCR) | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU29740 ccpA] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/CcpA CcpA] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/CcpA CcpA] | ||
| Line 26: | Line 26: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[motP]]'', ''[[aroA]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[motP]]'', ''[[aroA]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU29740 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU29740 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU29740 Advanced_DNA] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:ccpA_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:ccpA_context.gif]] | ||
Revision as of 13:37, 13 May 2013
- Description: Carbon catabolite control protein A, involved in glucose regulation of many genes; represses catabolic genes and activates genes involved in excretion of excess carbon
| Gene name | ccpA |
| Synonyms | graR, alsA, amyR |
| Essential | no |
| Product | transcriptional regulator (LacI family) |
| Function | mediates carbon catabolite repression (CCR) |
| Gene expression levels in SubtiExpress: ccpA | |
| Interactions involving this protein in SubtInteract: CcpA | |
| Metabolic function and regulation of this protein in SubtiPathways: Nucleoside catabolism, Nucleotides (regulation), Ile, Leu, Val, His, Coenzyme A, Central C-metabolism | |
| MW, pI | 36,8 kDa, 5.06 |
| Gene length, protein length | 1002 bp, 334 amino acids |
| Immediate neighbours | motP, aroA |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 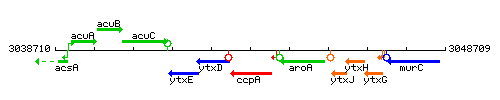
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
[hide]- 1 Categories containing this gene/protein
- 2 This gene is a member of the following regulons
- 3 The CcpA regulon
- 4 The gene
- 5 The protein
- 6 Expression and regulation
- 7 Biological materials
- 8 Labs working on this gene/protein
- 9 Your additional remarks
- 10 References
- 10.1 Reviews
- 10.2 General and physiological studies
- 10.3 Global analyses (proteome, transcriptome, ChIP-chip)
- 10.4 Repression of target genes by CcpA
- 10.5 Positive regulation of gene expression by CcpA
- 10.6 Control of CcpA activity
- 10.7 CcpA-DNA interaction
- 10.8 Functional analysis of CcpA
- 10.9 Structural analyses
Categories containing this gene/protein
- see also: glutamate metabolism
This gene is a member of the following regulons
The CcpA regulon
The gene
Basic information
- Locus tag: BSU29740
Phenotypes of a mutant
Loss of carbon catabolite repression. Loss of PTS-dependent sugar transport due to excessive phosphorylation of HPr by HprK. The mutant is unable to grow on a minimal medium with glucose and ammonium as the only sources of carbon and nitrogen, respectively.
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcriptional regulator of carbon catabolite repression (CCR)
- Protein family: LacI family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- HTH LacI-type Domain (1 – 58)
- DNA binding Domain (6 – 25)
- Modification:
- Effectors of protein activity:glucose-6-phosphate, fructose-1,6-bisphosphate Pubmed
Database entries
- Structure:
- 2JCG (Apoprotein from Bacillus megaterium)
- CcpA-Crh-DNA-complex NCBI
- complex with P-Ser-HPr and sulphate ions NCBI
- 3OQM (complex of B. subtilis CcpA with P-Ser-HPr and the ackA operator site)
- 3OQN (complex of B. subtilis CcpA with P-Ser-HPr and the gntR operator site)
- 3OQO (complex of B. subtilis CcpA with P-Ser-HPr and a optimal synthetic operator site)
- UniProt: P25144
- KEGG entry: [3]
Additional information
Expression and regulation
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Additional information: there are about 3.000 molecules of CcpA per cell PubMed, this corresponds to a concentration of 3 myM (according to PubMed)
Biological materials
- Mutant:
- QB5407 (spc), available in Jörg Stülke's lab
- GP302 (erm), available in Jörg Stülke's lab
- GP300 (an in frame deletion of ccpA), available in Jörg Stülke's lab
- WH649 (aphA3), available in Gerald Seidel's lab
- Expression vector:
- pGP643 (N-terminal Strep-tag, purification from B. subtilis, for SPINE, in pGP380), available in Jörg Stülke's lab
- pWH940 (C-terminal Strep-tag, purification from B. subtilis, for SPINE, in pGP382), available in Gerald Seidel's lab
- Strep-tag construct: GP1303 ccpA-Strep (spc) in native locus, based on (pGP1389), available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- Antibody: available in Gerald Seidel's and in Jörg Stülke's lab
Labs working on this gene/protein
- Gerald Seidel, Erlangen University, Germany Homepage
- Richard Brennan, Houston, Texas, USA Homepage
- Milton H. Saier, University of California at San Diego, USA Homepage
- Yasutaro Fujita, University of Fukuyama, Japan
- Jörg Stülke, University of Göttingen, Germany Homepage
- Oscar Kuipers, University of Groningen, The Netherlands Homepage
Your additional remarks
References
Reviews
Sabine Brantl, Andreas Licht
Characterisation of Bacillus subtilis transcriptional regulators involved in metabolic processes.
Curr Protein Pept Sci: 2010, 11(4);274-91
[PubMed:20408793]
[WorldCat.org]
[DOI]
(I p)
Yasutaro Fujita
Carbon catabolite control of the metabolic network in Bacillus subtilis.
Biosci Biotechnol Biochem: 2009, 73(2);245-59
[PubMed:19202299]
[WorldCat.org]
[DOI]
(I p)
Boris Görke, Jörg Stülke
Carbon catabolite repression in bacteria: many ways to make the most out of nutrients.
Nat Rev Microbiol: 2008, 6(8);613-24
[PubMed:18628769]
[WorldCat.org]
[DOI]
(I p)
Josef Deutscher
The mechanisms of carbon catabolite repression in bacteria.
Curr Opin Microbiol: 2008, 11(2);87-93
[PubMed:18359269]
[WorldCat.org]
[DOI]
(P p)
Jessica B Warner, Juke S Lolkema
CcpA-dependent carbon catabolite repression in bacteria.
Microbiol Mol Biol Rev: 2003, 67(4);475-90
[PubMed:14665673]
[WorldCat.org]
[DOI]
(P p)
T M Henkin
The role of CcpA transcriptional regulator in carbon metabolism in Bacillus subtilis.
FEMS Microbiol Lett: 1996, 135(1);9-15
[PubMed:8598282]
[WorldCat.org]
[DOI]
(P p)
General and physiological studies
Additional publications: PubMed
Global analyses (proteome, transcriptome, ChIP-chip)
Additional publications: PubMed
Repression of target genes by CcpA
Additional publications: PubMed
José Manuel Inácio, Isabel de Sá-Nogueira
trans-Acting factors and cis elements involved in glucose repression of arabinan degradation in Bacillus subtilis.
J Bacteriol: 2007, 189(22);8371-6
[PubMed:17827291]
[WorldCat.org]
[DOI]
(I p)
Soo-Keun Choi, Milton H Saier
Mechanism of CcpA-mediated glucose repression of the resABCDE operon of Bacillus subtilis.
J Mol Microbiol Biotechnol: 2006, 11(1-2);104-10
[PubMed:16825793]
[WorldCat.org]
[DOI]
(P p)
Soo-Keun Choi, Milton H Saier
Regulation of pho regulon gene expression by the carbon control protein A, CcpA, in Bacillus subtilis.
J Mol Microbiol Biotechnol: 2005, 10(1);40-50
[PubMed:16491025]
[WorldCat.org]
[DOI]
(P p)
Soo-Keun Choi, Milton H Saier
Regulation of sigL expression by the catabolite control protein CcpA involves a roadblock mechanism in Bacillus subtilis: potential connection between carbon and nitrogen metabolism.
J Bacteriol: 2005, 187(19);6856-61
[PubMed:16166551]
[WorldCat.org]
[DOI]
(P p)
Boris R Belitsky, Hyun-Jin Kim, Abraham L Sonenshein
CcpA-dependent regulation of Bacillus subtilis glutamate dehydrogenase gene expression.
J Bacteriol: 2004, 186(11);3392-8
[PubMed:15150224]
[WorldCat.org]
[DOI]
(P p)
Hyun-Jin Kim, Agnes Roux, Abraham L Sonenshein
Direct and indirect roles of CcpA in regulation of Bacillus subtilis Krebs cycle genes.
Mol Microbiol: 2002, 45(1);179-90
[PubMed:12100558]
[WorldCat.org]
[DOI]
(P p)
Hyun-Jin Kim, Cécile Jourlin-Castelli, Sam-In Kim, Abraham L Sonenshein
Regulation of the bacillus subtilis ccpC gene by ccpA and ccpC.
Mol Microbiol: 2002, 43(2);399-410
[PubMed:11985717]
[WorldCat.org]
[DOI]
(P p)
Emmanuelle Darbon, Pascale Servant, Sandrine Poncet, Josef Deutscher
Antitermination by GlpP, catabolite repression via CcpA and inducer exclusion triggered by P-GlpK dephosphorylation control Bacillus subtilis glpFK expression.
Mol Microbiol: 2002, 43(4);1039-52
[PubMed:11929549]
[WorldCat.org]
[DOI]
(P p)
I Martin-Verstraete, J Stülke, A Klier, G Rapoport
Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon.
J Bacteriol: 1995, 177(23);6919-27
[PubMed:7592486]
[WorldCat.org]
[DOI]
(P p)
F J Grundy, A J Turinsky, T M Henkin
Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA.
J Bacteriol: 1994, 176(15);4527-33
[PubMed:7913927]
[WorldCat.org]
[DOI]
(P p)
Positive regulation of gene expression by CcpA
Robert P Shivers, Abraham L Sonenshein
Bacillus subtilis ilvB operon: an intersection of global regulons.
Mol Microbiol: 2005, 56(6);1549-59
[PubMed:15916605]
[WorldCat.org]
[DOI]
(P p)
Holger Ludwig, Christoph Meinken, Anastasija Matin, Jörg Stülke
Insufficient expression of the ilv-leu operon encoding enzymes of branched-chain amino acid biosynthesis limits growth of a Bacillus subtilis ccpA mutant.
J Bacteriol: 2002, 184(18);5174-8
[PubMed:12193635]
[WorldCat.org]
[DOI]
(P p)
A J Turinsky, T R Moir-Blais, F J Grundy, T M Henkin
Bacillus subtilis ccpA gene mutants specifically defective in activation of acetoin biosynthesis.
J Bacteriol: 2000, 182(19);5611-4
[PubMed:10986270]
[WorldCat.org]
[DOI]
(P p)
E Presecan-Siedel, A Galinier, R Longin, J Deutscher, A Danchin, P Glaser, I Martin-Verstraete
Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis.
J Bacteriol: 1999, 181(22);6889-97
[PubMed:10559153]
[WorldCat.org]
[DOI]
(P p)
A J Turinsky, F J Grundy, J H Kim, G H Chambliss, T M Henkin
Transcriptional activation of the Bacillus subtilis ackA gene requires sequences upstream of the promoter.
J Bacteriol: 1998, 180(22);5961-7
[PubMed:9811655]
[WorldCat.org]
[DOI]
(P p)
F J Grundy, D A Waters, S H Allen, T M Henkin
Regulation of the Bacillus subtilis acetate kinase gene by CcpA.
J Bacteriol: 1993, 175(22);7348-55
[PubMed:8226682]
[WorldCat.org]
[DOI]
(P p)
Control of CcpA activity
CcpA-DNA interaction
Functional analysis of CcpA
H Ludwig, J Stülke
The Bacillus subtilis catabolite control protein CcpA exerts all its regulatory functions by DNA-binding.
FEMS Microbiol Lett: 2001, 203(1);125-9
[PubMed:11557150]
[WorldCat.org]
[DOI]
(P p)
E Küster-Schöck, A Wagner, U Völker, W Hillen
Mutations in catabolite control protein CcpA showing glucose-independent regulation in Bacillus megaterium.
J Bacteriol: 1999, 181(24);7634-8
[PubMed:10601226]
[WorldCat.org]
[DOI]
(P p)
E Küster, T Hilbich, M K Dahl, W Hillen
Mutations in catabolite control protein CcpA separating growth effects from catabolite repression.
J Bacteriol: 1999, 181(13);4125-8
[PubMed:10383986]
[WorldCat.org]
[DOI]
(P p)
A Kraus, E Küster, A Wagner, K Hoffmann, W Hillen
Identification of a co-repressor binding site in catabolite control protein CcpA.
Mol Microbiol: 1998, 30(5);955-63
[PubMed:9988473]
[WorldCat.org]
[DOI]
(P p)
A Kraus, W Hillen
Analysis of CcpA mutations defective in carbon catabolite repression in Bacillus megaterium.
FEMS Microbiol Lett: 1997, 153(1);221-6
[PubMed:9252590]
[WorldCat.org]
[DOI]
(P p)
Structural analyses
Bernhard Loll, Wolfram Saenger, Jacek Biesiadka
Structure of full-length transcription regulator CcpA in the apo form.
Biochim Biophys Acta: 2007, 1774(6);732-6
[PubMed:17500051]
[WorldCat.org]
[DOI]
(P p)
Rajesh Kumar Singh, Gottfried J Palm, Santosh Panjikar, Winfried Hinrichs
Structure of the apo form of the catabolite control protein A (CcpA) from Bacillus megaterium with a DNA-binding domain.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2007, 63(Pt 4);253-7
[PubMed:17401189]
[WorldCat.org]
[DOI]
(I p)
Maria A Schumacher, Gerald Seidel, Wolfgang Hillen, Richard G Brennan
Structural mechanism for the fine-tuning of CcpA function by the small molecule effectors glucose 6-phosphate and fructose 1,6-bisphosphate.
J Mol Biol: 2007, 368(4);1042-50
[PubMed:17376479]
[WorldCat.org]
[DOI]
(P p)
Vincent Chaptal, Virginie Gueguen-Chaignon, Sandrine Poncet, Cécile Lecampion, Philippe Meyer, Josef Deutscher, Anne Galinier, Sylvie Nessler, Solange Moréra
Structural analysis of B. subtilis CcpA effector binding site.
Proteins: 2006, 64(3);814-6
[PubMed:16755587]
[WorldCat.org]
[DOI]
(I p)
Maria A Schumacher, Gerald Seidel, Wolfgang Hillen, Richard G Brennan
Phosphoprotein Crh-Ser46-P displays altered binding to CcpA to effect carbon catabolite regulation.
J Biol Chem: 2006, 281(10);6793-800
[PubMed:16316990]
[WorldCat.org]
[DOI]
(P p)
Maria A Schumacher, Gregory S Allen, Marco Diel, Gerald Seidel, Wolfgang Hillen, Richard G Brennan
Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P.
Cell: 2004, 118(6);731-41
[PubMed:15369672]
[WorldCat.org]
[DOI]
(P p)
J Tebbe, P Orth, E K Küster-Schöck, W Hillen, W Saenger, W Hinrichs
Crystallization and preliminary X-ray analyses of catabolite control protein A, free and in complex with its DNA-binding site.
Acta Crystallogr D Biol Crystallogr: 2000, 56(Pt 1);67-9
[PubMed:10666630]
[WorldCat.org]
[DOI]
(P p)