Difference between revisions of "PolC"
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || [[DNA replication]] | |style="background:#ABCDEF;" align="center"|'''Function''' || [[DNA replication]] | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU16580 polC] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/PolC PolC] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/PolC PolC] | ||
| Line 24: | Line 24: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[proS]]'', ''[[ylxS]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[proS]]'', ''[[ylxS]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU16580 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU16580 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU16580 Advanced_DNA] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:polC_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:polC_context.gif]] | ||
Revision as of 12:51, 13 May 2013
- Description: DNA polymerase III (alpha subunit), part of the replisome
| Gene name | polC |
| Synonyms | mutI, dnaF, dnaP |
| Essential | yes PubMed |
| Product | DNA polymerase III (alpha subunit) |
| Function | DNA replication |
| Gene expression levels in SubtiExpress: polC | |
| Interactions involving this protein in SubtInteract: PolC | |
| MW, pI | 162 kDa, 5.135 |
| Gene length, protein length | 4311 bp, 1437 aa |
| Immediate neighbours | proS, ylxS |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed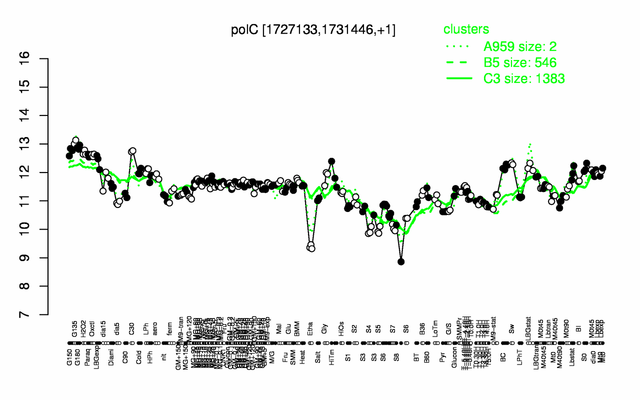
| |
Contents
Categories containing this gene/protein
DNA replication, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16580
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1) (according to Swiss-Prot)
- required for bacteriophage SPP1 replication PubMed
- Protein family: PolC subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- forms foci PubMed
- cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: P13267
- KEGG entry: [2]
- E.C. number: 2.7.7.7
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Olivier Rannou, Emmanuelle Le Chatelier, Marilynn A Larson, Hamid Nouri, Bérengère Dalmais, Charles Laughton, Laurent Jannière, Panos Soultanas
Functional interplay of DnaE polymerase, DnaG primase and DnaC helicase within a ternary complex, and primase to polymerase hand-off during lagging strand DNA replication in Bacillus subtilis.
Nucleic Acids Res: 2013, 41(10);5303-20
[PubMed:23563155]
[WorldCat.org]
[DOI]
(I p)
Elena M Seco, John C Zinder, Carol M Manhart, Ambra Lo Piano, Charles S McHenry, Silvia Ayora
Bacteriophage SPP1 DNA replication strategies promote viral and disable host replication in vitro.
Nucleic Acids Res: 2013, 41(3);1711-21
[PubMed:23268446]
[WorldCat.org]
[DOI]
(I p)
Andrew D Klocko, Jeremy W Schroeder, Brian W Walsh, Justin S Lenhart, Margery L Evans, Lyle A Simmons
Mismatch repair causes the dynamic release of an essential DNA polymerase from the replication fork.
Mol Microbiol: 2011, 82(3);648-63
[PubMed:21958350]
[WorldCat.org]
[DOI]
(I p)
Kęstutis Timinskas, Česlovas Venclovas
The N-terminal region of the bacterial DNA polymerase PolC features a pair of domains, both distantly related to domain V of the DNA polymerase III τ subunit.
FEBS J: 2011, 278(17);3109-18
[PubMed:21740522]
[WorldCat.org]
[DOI]
(I p)
Glenn M Sanders, H Garry Dallmann, Charles S McHenry
Reconstitution of the B. subtilis replisome with 13 proteins including two distinct replicases.
Mol Cell: 2010, 37(2);273-81
[PubMed:20122408]
[WorldCat.org]
[DOI]
(I p)
Megan E Rokop, Jennifer M Auchtung, Alan D Grossman
Control of DNA replication initiation by recruitment of an essential initiation protein to the membrane of Bacillus subtilis.
Mol Microbiol: 2004, 52(6);1757-67
[PubMed:15186423]
[WorldCat.org]
[DOI]
(P p)
E Dervyn, C Suski, R Daniel, C Bruand, J Chapuis, J Errington, L Jannière, S D Ehrlich
Two essential DNA polymerases at the bacterial replication fork.
Science: 2001, 294(5547);1716-9
[PubMed:11721055]
[WorldCat.org]
[DOI]
(P p)