Difference between revisions of "CcpA"
(→References) |
|||
| Line 171: | Line 171: | ||
==Global analyses (proteome, transcriptome, ChIP-chip)== | ==Global analyses (proteome, transcriptome, ChIP-chip)== | ||
| − | <pubmed>12850135 ,11251851,10559165, 11160890,17183215 22383848 | + | '''Additional publications:''' {{PubMed|22900538}} |
| + | <pubmed>12850135 ,11251851,10559165, 11160890,17183215 22383848 </pubmed> | ||
==Repression of target genes by CcpA== | ==Repression of target genes by CcpA== | ||
Revision as of 16:22, 30 October 2012
- Description: Carbon catabolite control protein A, involved in glucose regulation of many genes; represses catabolic genes and activates genes involved in excretion of excess carbon
| Gene name | ccpA |
| Synonyms | graR, alsA, amyR |
| Essential | no |
| Product | transcriptional regulator (LacI family) |
| Function | mediates carbon catabolite repression (CCR) |
| Gene expression levels in SubtiExpress: ccpA | |
| Interactions involving this protein in SubtInteract: CcpA | |
| Metabolic function and regulation of this protein in SubtiPathways: Nucleoside catabolism, Nucleotides (regulation), Ile, Leu, Val, His, Coenzyme A, Central C-metabolism | |
| MW, pI | 36,8 kDa, 5.06 |
| Gene length, protein length | 1002 bp, 334 amino acids |
| Immediate neighbours | motP, aroA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 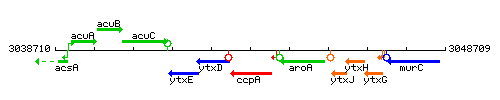
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
[hide]- 1 Categories containing this gene/protein
- 2 This gene is a member of the following regulons
- 3 The CcpA regulon
- 4 The gene
- 5 The protein
- 6 Expression and regulation
- 7 Biological materials
- 8 Labs working on this gene/protein
- 9 Your additional remarks
- 10 References
- 10.1 Reviews
- 10.2 General and physiological studies
- 10.3 Global analyses (proteome, transcriptome, ChIP-chip)
- 10.4 Repression of target genes by CcpA
- 10.5 Positive regulation of gene expression by CcpA
- 10.6 Control of CcpA activity
- 10.7 CcpA-DNA interaction
- 10.8 Functional analysis of CcpA
- 10.9 Structural analyses
Categories containing this gene/protein
- see also: glutamate metabolism
This gene is a member of the following regulons
The CcpA regulon
The gene
Basic information
- Locus tag: BSU29740
Phenotypes of a mutant
Loss of carbon catabolite repression. Loss of PTS-dependent sugar transport due to excessive phosphorylation of HPr by HprK. The mutant is unable to grow on a minimal medium with glucose and ammonium as the only sources of carbon and nitrogen, respectively.
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcriptional regulator of carbon catabolite repression (CCR)
- Protein family: LacI family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- HTH LacI-type Domain (1 – 58)
- DNA binding Domain (6 – 25)
- Modification:
- Effectors of protein activity:glucose-6-phosphate, fructose-1,6-bisphosphate Pubmed
Database entries
- Structure:
- 2JCG (Apoprotein from Bacillus megaterium)
- CcpA-Crh-DNA-complex NCBI
- complex with P-Ser-HPr and sulphate ions NCBI
- 3OQM (complex of B. subtilis CcpA with P-Ser-HPr and the ackA operator site)
- 3OQN (complex of B. subtilis CcpA with P-Ser-HPr and the gntR operator site)
- 3OQO (complex of B. subtilis CcpA with P-Ser-HPr and a optimal synthetic operator site)
- UniProt: P25144
- KEGG entry: [3]
Additional information
Expression and regulation
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Additional information: there are about 3.000 molecules of CcpA per cell PubMed, this corresponds to a concentration of 3 myM (according to PubMed)
Biological materials
- Mutant:
- QB5407 (spc), available in Jörg Stülke's lab
- GP302 (erm), available in Jörg Stülke's lab
- GP300 (an in frame deletion of ccpA), available in Jörg Stülke's lab
- WH649 (aphA3), available in Gerald Seidel's lab
- Expression vector:
- pGP643 (N-terminal Strep-tag, purification from B. subtilis, for SPINE, in pGP380), available in Jörg Stülke's lab
- pWH940 (C-terminal Strep-tag, purification from B. subtilis, for SPINE, in pGP382), available in Gerald Seidel's lab
- Strep-tag construct: GP1303 ccpA-Strep (spc) in native locus, based on (pGP1389), available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- Antibody: available in Gerald Seidel's and in Jörg Stülke's lab
Labs working on this gene/protein
- Gerald Seidel, Erlangen University, Germany Homepage
- Richard Brennan, Houston, Texas, USA Homepage
- Milton H. Saier, University of California at San Diego, USA Homepage
- Yasutaro Fujita, University of Fukuyama, Japan
- Jörg Stülke, University of Göttingen, Germany Homepage
- Oscar Kuipers, University of Groningen, The Netherlands Homepage
Your additional remarks
References
Reviews
General and physiological studies
Additional publications: PubMed
Frederik M Meyer, Matthieu Jules, Felix M P Mehne, Dominique Le Coq, Jens J Landmann, Boris Görke, Stéphane Aymerich, Jörg Stülke
Malate-mediated carbon catabolite repression in Bacillus subtilis involves the HPrK/CcpA pathway.
J Bacteriol: 2011, 193(24);6939-49
[PubMed:22001508]
[WorldCat.org]
[DOI]
(I p)
Kalpana D Singh, Matthias H Schmalisch, Jörg Stülke, Boris Görke
Carbon catabolite repression in Bacillus subtilis: quantitative analysis of repression exerted by different carbon sources.
J Bacteriol: 2008, 190(21);7275-84
[PubMed:18757537]
[WorldCat.org]
[DOI]
(I p)
Naoya Terahara, Makoto Fujisawa, Benjamin Powers, Tina M Henkin, Terry A Krulwich, Masahiro Ito
An intergenic stem-loop mutation in the Bacillus subtilis ccpA-motPS operon increases motPS transcription and the MotPS contribution to motility.
J Bacteriol: 2006, 188(7);2701-5
[PubMed:16547058]
[WorldCat.org]
[DOI]
(P p)
Ingrid Wacker, Holger Ludwig, Irene Reif, Hans-Matti Blencke, Christian Detsch, Jörg Stülke
The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA.
Microbiology (Reading): 2003, 149(Pt 10);3001-3009
[PubMed:14523131]
[WorldCat.org]
[DOI]
(P p)
Holger Ludwig, Nicole Rebhan, Hans-Matti Blencke, Matthias Merzbacher, Jörg Stülke
Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation.
Mol Microbiol: 2002, 45(2);543-53
[PubMed:12123463]
[WorldCat.org]
[DOI]
(P p)
N Faires, S Tobisch, S Bachem, I Martin-Verstraete, M Hecker, J Stülke
The catabolite control protein CcpA controls ammonium assimilation in Bacillus subtilis.
J Mol Microbiol Biotechnol: 1999, 1(1);141-8
[PubMed:10941796]
[WorldCat.org]
(P p)
Y Miwa, M Saikawa, Y Fujita
Possible function and some properties of the CcpA protein of Bacillus subtilis.
Microbiology (Reading): 1994, 140 ( Pt 10);2567-75
[PubMed:8000527]
[WorldCat.org]
[DOI]
(P p)
T M Henkin, F J Grundy, W L Nicholson, G H Chambliss
Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacl and galR repressors.
Mol Microbiol: 1991, 5(3);575-84
[PubMed:1904524]
[WorldCat.org]
[DOI]
(P p)
Global analyses (proteome, transcriptome, ChIP-chip)
Additional publications: PubMed
Joerg Martin Buescher, Wolfram Liebermeister, Matthieu Jules, Markus Uhr, Jan Muntel, Eric Botella, Bernd Hessling, Roelco Jacobus Kleijn, Ludovic Le Chat, François Lecointe, Ulrike Mäder, Pierre Nicolas, Sjouke Piersma, Frank Rügheimer, Dörte Becher, Philippe Bessieres, Elena Bidnenko, Emma L Denham, Etienne Dervyn, Kevin M Devine, Geoff Doherty, Samuel Drulhe, Liza Felicori, Mark J Fogg, Anne Goelzer, Annette Hansen, Colin R Harwood, Michael Hecker, Sebastian Hubner, Claus Hultschig, Hanne Jarmer, Edda Klipp, Aurélie Leduc, Peter Lewis, Frank Molina, Philippe Noirot, Sabine Peres, Nathalie Pigeonneau, Susanne Pohl, Simon Rasmussen, Bernd Rinn, Marc Schaffer, Julian Schnidder, Benno Schwikowski, Jan Maarten Van Dijl, Patrick Veiga, Sean Walsh, Anthony J Wilkinson, Jörg Stelling, Stéphane Aymerich, Uwe Sauer
Global network reorganization during dynamic adaptations of Bacillus subtilis metabolism.
Science: 2012, 335(6072);1099-103
[PubMed:22383848]
[WorldCat.org]
[DOI]
(I p)
Andrzej T Lulko, Girbe Buist, Jan Kok, Oscar P Kuipers
Transcriptome analysis of temporal regulation of carbon metabolism by CcpA in Bacillus subtilis reveals additional target genes.
J Mol Microbiol Biotechnol: 2007, 12(1-2);82-95
[PubMed:17183215]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
M S Moreno, B L Schneider, R R Maile, W Weyler, M H Saier
Catabolite repression mediated by the CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses.
Mol Microbiol: 2001, 39(5);1366-81
[PubMed:11251851]
[WorldCat.org]
[DOI]
(P p)
K Yoshida, K Kobayashi, Y Miwa, C M Kang, M Matsunaga, H Yamaguchi, S Tojo, M Yamamoto, R Nishi, N Ogasawara, T Nakayama, Y Fujita
Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis.
Nucleic Acids Res: 2001, 29(3);683-92
[PubMed:11160890]
[WorldCat.org]
[DOI]
(I p)
S Tobisch, D Zühlke, J Bernhardt, J Stülke, M Hecker
Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis.
J Bacteriol: 1999, 181(22);6996-7004
[PubMed:10559165]
[WorldCat.org]
[DOI]
(P p)
Repression of target genes by CcpA
Additional publications: PubMed
Positive regulation of gene expression by CcpA
Control of CcpA activity
Lwin Mar Aung-Hilbrich, Gerald Seidel, Andrea Wagner, Wolfgang Hillen
Quantification of the influence of HPrSer46P on CcpA-cre interaction.
J Mol Biol: 2002, 319(1);77-85
[PubMed:12051938]
[WorldCat.org]
[DOI]
(P p)
A Galinier, J Deutscher, I Martin-Verstraete
Phosphorylation of either crh or HPr mediates binding of CcpA to the bacillus subtilis xyn cre and catabolite repression of the xyn operon.
J Mol Biol: 1999, 286(2);307-14
[PubMed:9973552]
[WorldCat.org]
[DOI]
(P p)
J H Kim, M I Voskuil, G H Chambliss
NADP, corepressor for the Bacillus catabolite control protein CcpA.
Proc Natl Acad Sci U S A: 1998, 95(16);9590-5
[PubMed:9689125]
[WorldCat.org]
[DOI]
(P p)
B E Jones, V Dossonnet, E Küster, W Hillen, J Deutscher, R E Klevit
Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr.
J Biol Chem: 1997, 272(42);26530-5
[PubMed:9334231]
[WorldCat.org]
[DOI]
(P p)
J Deutscher, E Küster, U Bergstedt, V Charrier, W Hillen
Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria.
Mol Microbiol: 1995, 15(6);1049-53
[PubMed:7623661]
[WorldCat.org]
[DOI]
(P p)
CcpA-DNA interaction
Maria A Schumacher, Mareen Sprehe, Maike Bartholomae, Wolfgang Hillen, Richard G Brennan
Structures of carbon catabolite protein A-(HPr-Ser46-P) bound to diverse catabolite response element sites reveal the basis for high-affinity binding to degenerate DNA operators.
Nucleic Acids Res: 2011, 39(7);2931-42
[PubMed:21106498]
[WorldCat.org]
[DOI]
(I p)
Gerald Seidel, Marco Diel, Norbert Fuchsbauer, Wolfgang Hillen
Quantitative interdependence of coeffectors, CcpA and cre in carbon catabolite regulation of Bacillus subtilis.
FEBS J: 2005, 272(10);2566-77
[PubMed:15885105]
[WorldCat.org]
[DOI]
(P p)
Y Miwa, A Nakata, A Ogiwara, M Yamamoto, Y Fujita
Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis.
Nucleic Acids Res: 2000, 28(5);1206-10
[PubMed:10666464]
[WorldCat.org]
[DOI]
(I p)
J H Kim, G H Chambliss
Contacts between Bacillus subtilis catabolite regulatory protein CcpA and amyO target site.
Nucleic Acids Res: 1997, 25(17);3490-6
[PubMed:9254709]
[WorldCat.org]
[DOI]
(P p)
Y Fujita, Y Miwa, A Galinier, J Deutscher
Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr.
Mol Microbiol: 1995, 17(5);953-60
[PubMed:8596444]
[WorldCat.org]
[DOI]
(P p)
J H Kim, Z T Guvener, J Y Cho, K C Chung, G H Chambliss
Specificity of DNA binding activity of the Bacillus subtilis catabolite control protein CcpA.
J Bacteriol: 1995, 177(17);5129-34
[PubMed:7665492]
[WorldCat.org]
[DOI]
(P p)
Functional analysis of CcpA
H Ludwig, J Stülke
The Bacillus subtilis catabolite control protein CcpA exerts all its regulatory functions by DNA-binding.
FEMS Microbiol Lett: 2001, 203(1);125-9
[PubMed:11557150]
[WorldCat.org]
[DOI]
(P p)
E Küster-Schöck, A Wagner, U Völker, W Hillen
Mutations in catabolite control protein CcpA showing glucose-independent regulation in Bacillus megaterium.
J Bacteriol: 1999, 181(24);7634-8
[PubMed:10601226]
[WorldCat.org]
[DOI]
(P p)
E Küster, T Hilbich, M K Dahl, W Hillen
Mutations in catabolite control protein CcpA separating growth effects from catabolite repression.
J Bacteriol: 1999, 181(13);4125-8
[PubMed:10383986]
[WorldCat.org]
[DOI]
(P p)
A Kraus, E Küster, A Wagner, K Hoffmann, W Hillen
Identification of a co-repressor binding site in catabolite control protein CcpA.
Mol Microbiol: 1998, 30(5);955-63
[PubMed:9988473]
[WorldCat.org]
[DOI]
(P p)
A Kraus, W Hillen
Analysis of CcpA mutations defective in carbon catabolite repression in Bacillus megaterium.
FEMS Microbiol Lett: 1997, 153(1);221-6
[PubMed:9252590]
[WorldCat.org]
[DOI]
(P p)
Structural analyses