Difference between revisions of "YrvO"
| Line 1: | Line 1: | ||
| − | * '''Description:''' cysteine desulfurase<br/><br/> | + | * '''Description:''' cysteine desulfurase involved in tRNA thiolation<br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 12: | Line 12: | ||
|style="background:#ABCDEF;" align="center"| '''Product''' || cysteine desulfurase | |style="background:#ABCDEF;" align="center"| '''Product''' || cysteine desulfurase | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || | + | |style="background:#ABCDEF;" align="center"|'''Function''' || tRNA modification |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 41 kDa, 5.463 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 41 kDa, 5.463 | ||
| Line 42: | Line 42: | ||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
{{SubtiWiki category|[[essential genes]]}}, | {{SubtiWiki category|[[essential genes]]}}, | ||
| − | {{SubtiWiki category|[[ | + | {{SubtiWiki category|[[translation]]}}, |
{{SubtiWiki category|[[phosphoproteins]]}} | {{SubtiWiki category|[[phosphoproteins]]}} | ||
| Line 138: | Line 138: | ||
=References= | =References= | ||
| − | <pubmed>16885442 11948165 10715213 22517742</pubmed> | + | <pubmed>16885442 11948165 10715213 22517742 12682299 17064282</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:12, 21 April 2012
- Description: cysteine desulfurase involved in tRNA thiolation
| Gene name | yrvO |
| Synonyms | nifS, iscS |
| Essential | yes PubMed |
| Product | cysteine desulfurase |
| Function | tRNA modification |
| MW, pI | 41 kDa, 5.463 |
| Gene length, protein length | 1137 bp, 379 aa |
| Immediate neighbours | trmU, cymR |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 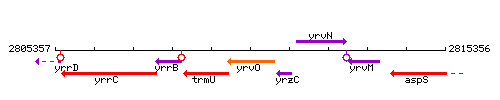
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
essential genes, translation, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU27510
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-385 PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: O34599
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References