Difference between revisions of "SdpC"
| Line 1: | Line 1: | ||
| − | * '''Description:''' toxin, kills non-sporulating cells, induces activity of [[SigW]] <br/><br/> | + | * '''Description:''' toxin, collapses the proton motive force and induces autolysis, kills non-sporulating cells, induces activity of [[SigW]] <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 65: | Line 65: | ||
* '''Catalyzed reaction/ biological activity:''' | * '''Catalyzed reaction/ biological activity:''' | ||
| + | ** toxin, collapses the proton motive force and induces autolysis {{PubMed|22469514}} | ||
* '''Protein family:''' | * '''Protein family:''' | ||
| Line 141: | Line 142: | ||
<pubmed>20955377 </pubmed> | <pubmed>20955377 </pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| + | '''Additional publications:''' {{PubMed|20805502}} | ||
| + | <pubmed> 22469514 </pubmed> | ||
<big>''Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J'' </big> | <big>''Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J'' </big> | ||
<big>'''RNA processing in ''Bacillus subtilis'': identification of targets of the essential RNase Y.''' </big> | <big>'''RNA processing in ''Bacillus subtilis'': identification of targets of the essential RNase Y.''' </big> | ||
| Line 146: | Line 149: | ||
[http://www.ncbi.nlm.nih.gov/pubmed/21815947 PubMed:21815947] | [http://www.ncbi.nlm.nih.gov/pubmed/21815947 PubMed:21815947] | ||
<pubmed>,12817086,16469701,16629676,13129613,14651647,15687200,15743949,15687200,17720793,12107147, </pubmed> | <pubmed>,12817086,16469701,16629676,13129613,14651647,15687200,15743949,15687200,17720793,12107147, </pubmed> | ||
| − | + | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:17, 4 April 2012
- Description: toxin, collapses the proton motive force and induces autolysis, kills non-sporulating cells, induces activity of SigW
| Gene name | sdpC |
| Synonyms | yvaY |
| Essential | no |
| Product | toxin, kills non-sporulating cells |
| Function | killing of non-sporulating sister cells |
| Interactions involving this protein in SubtInteract: SdpC | |
| MW, pI | 22 kDa, 9.611 |
| Gene length, protein length | 609 bp, 203 aa |
| Immediate neighbours | sdpB, sdpI |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 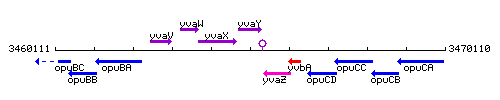
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
toxins, antitoxins and immunity against toxins
This gene is a member of the following regulons
AbrB regulon, Rok regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU33770
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- toxin, collapses the proton motive force and induces autolysis PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: the meture SDP is a 42-residue peptide with one disulfide bridge PubMed
- Cofactor(s):
- Effectors of protein activity:
- Localization: secreted (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O34344
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original Publications
Additional publications: PubMed
Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol. 2011 81(6): 1459-1473. PubMed:21815947