Difference between revisions of "Ung"
(→Extended information on the protein) |
|||
| Line 46: | Line 46: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| − | + | * increased mutation rates {{PubMed|22056936}} | |
=== Database entries === | === Database entries === | ||
| Line 101: | Line 101: | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| + | ** expressed throughout growth and staionary phase {{PubMed|22056936}} | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| Line 126: | Line 127: | ||
=References= | =References= | ||
'''Additional publications:''' {{PubMed|21170359,21542855}} | '''Additional publications:''' {{PubMed|21170359,21542855}} | ||
| − | <pubmed> 9353933 20693660</pubmed> | + | <pubmed> 9353933 20693660 22056936</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:31, 8 November 2011
- Description: uracil-DNA glycosylase
| Gene name | ung |
| Synonyms | ipa-57d, ywdG |
| Essential | no |
| Product | uracil-DNA glycosylase |
| Function | DNA repair |
| Interactions involving this protein in SubtInteract: Ung | |
| MW, pI | 25 kDa, 8.782 |
| Gene length, protein length | 675 bp, 225 aa |
| Immediate neighbours | ywdH, ywdF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 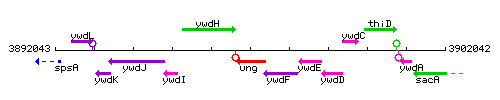
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU37970
Phenotypes of a mutant
- increased mutation rates PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: removes uracil preferentially from single-stranded DNA over double-stranded DNA, exhibiting higher preference for U:G than U:A mismatches PubMed
- Protein family: uracil-DNA glycosylase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: P39615
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: ung PubMed
- Regulation:
- expressed throughout growth and staionary phase PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Karina López-Olmos, Martha P Hernández, Jorge A Contreras-Garduño, Eduardo A Robleto, Peter Setlow, Ronald E Yasbin, Mario Pedraza-Reyes
Roles of endonuclease V, uracil-DNA glycosylase, and mismatch repair in Bacillus subtilis DNA base-deamination-induced mutagenesis.
J Bacteriol: 2012, 194(2);243-52
[PubMed:22056936]
[WorldCat.org]
[DOI]
(I p)
Prem Singh Kaushal, Ramappa K Talawar, Umesh Varshney, M Vijayan
Structure of uracil-DNA glycosylase from Mycobacterium tuberculosis: insights into interactions with ligands.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2010, 66(Pt 8);887-92
[PubMed:20693660]
[WorldCat.org]
[DOI]
(I p)
E Presecan, I Moszer, L Boursier, H Cruz Ramos, V de la Fuente, M-F Hullo, C Lelong, S Schleich, A Sekowska, B H Song, G Villani, F Kunst, A Danchin, P Glaser
The Bacillus subtilis genome from gerBC (311 degrees) to licR (334 degrees).
Microbiology (Reading): 1997, 143 ( Pt 10);3313-3328
[PubMed:9353933]
[WorldCat.org]
[DOI]
(P p)