Difference between revisions of "StoA"
| Line 53: | Line 53: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| Line 111: | Line 109: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** A [[ncRNA]] is predicted between ''[[stoA]]'' and ''[[zosA]]'' {{PubMed|20525796}} | ||
=Biological materials = | =Biological materials = | ||
| Line 135: | Line 134: | ||
<pubmed> 19919674 </pubmed> | <pubmed> 19919674 </pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| − | <pubmed>,12662922,15292147,15342593, 19144642 19919673 </pubmed> | + | <pubmed>,12662922,15292147,15342593, 19144642 19919673 20525796</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:31, 4 May 2011
- Description: thiol-disulfide oxidoreductase, with thioredoxin-like domain, required for the synthesis of the endospore peptidoglycan cortex
| Gene name | stoA |
| Synonyms | spoIVH, ykvV |
| Essential | no |
| Product | thiol-disulfide oxidoreductase |
| Function | spore cortex formation |
| MW, pI | 18 kDa, 5.297 |
| Gene length, protein length | 495 bp, 165 aa |
| Immediate neighbours | ykvU, zosA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 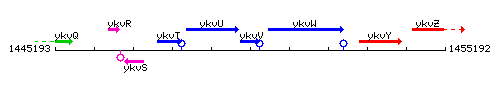
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU13840
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: reduction of intramolecular disulfide bonds in SpoVD PubMed
- Protein family: thioredoxin domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: forespore envelope PubMed
Database entries
- Structure: 3ERW
- UniProt: O31687
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Lars Hederstedt, University of Lund, Sweden Homepage
Your additional remarks
References
Reviews
Original Publications