Difference between revisions of "FtsL"
(→References) |
|||
| Line 72: | Line 72: | ||
* '''Effectors of protein activity:''' degraded by [[RasP]], stabilized against [[RasP]] cleavage by [[DivIC]] {{PubMed|20644139}} | * '''Effectors of protein activity:''' degraded by [[RasP]], stabilized against [[RasP]] cleavage by [[DivIC]] {{PubMed|20644139}} | ||
| − | * '''Interactions:''' [[RasP]]-[[FtsL]], [[DivIC]]-[[FtsL]] {{PubMed|10844672}} | + | * '''Interactions:''' |
| + | ** [[RasP]]-[[FtsL]], [[DivIC]]-[[FtsL]] {{PubMed|10844672}} | ||
| + | ** [[PbpB]]-[[DivIB]]-([[FtsL]]-[[DivIC]])2 {{PubMed|20870765}}, as part of the [[divisome]] | ||
* '''Localization:''' cell membrane (according to Swiss-Prot) | * '''Localization:''' cell membrane (according to Swiss-Prot) | ||
| Line 122: | Line 124: | ||
<pubmed> 20836086 </pubmed> | <pubmed> 20836086 </pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| − | <pubmed>9720875,10792716,16936019,16549676,17020588,8636036,10986263, 11994149, 10844672,19429628 20644139 </pubmed> | + | <pubmed>9720875,10792716,16936019,16549676,17020588,8636036,10986263, 11994149, 10844672,19429628 20644139 20870765</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:41, 28 September 2010
- Description: cell-division protein (septum formation), controls together with EzrA dynamics of the FtsZ ring
| Gene name | ftsL |
| Synonyms | ylxB, yllD |
| Essential | yes PubMed |
| Product | cell-division protein |
| Function | septum formation |
| MW, pI | 12 kDa, 10.083 |
| Gene length, protein length | 351 bp, 117 aa |
| Immediate neighbours | mraW, pbpB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 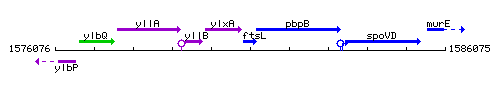
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU15150
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ftsL family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: Q07867
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews=
Original Publications