Difference between revisions of "ThiD"
(→Reviews) |
|||
| Line 123: | Line 123: | ||
=References= | =References= | ||
==Reviews== | ==Reviews== | ||
| − | <pubmed>19348578 </pubmed> | + | <pubmed>19348578 10382260 </pubmed> |
| + | |||
==Original publications== | ==Original publications== | ||
<pubmed>14973012,16978644 , </pubmed> | <pubmed>14973012,16978644 , </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:27, 11 August 2010
- Description: 4-amino-5-hydroxymethyl-2-methylpyrimidine and 4-amino-5-hydroxymethyl-2-methylpyrimidine pyrophosphate kinase
| Gene name | thiD |
| Synonyms | yjbV |
| Essential | no |
| Product | 4-amino-5-hydroxymethyl-2-methylpyrimidine pyrophosphate kinase |
| Function | biosynthesis of thiamine pyrophosphate (TPP) |
| Metabolic function and regulation of this protein in SubtiPathways: Thiamin | |
| MW, pI | 28 kDa, 5.709 |
| Gene length, protein length | 813 bp, 271 aa |
| Immediate neighbours | thiF, fabI |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 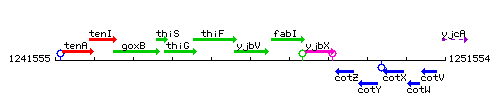
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU11710
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s): PdxK
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- UniProt: O31620
- KEGG entry: [3]
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Christopher T Jurgenson, Tadhg P Begley, Steven E Ealick
The structural and biochemical foundations of thiamin biosynthesis.
Annu Rev Biochem: 2009, 78;569-603
[PubMed:19348578]
[WorldCat.org]
[DOI]
(I p)
T P Begley, D M Downs, S E Ealick, F W McLafferty, A P Van Loon, S Taylor, N Campobasso, H J Chiu, C Kinsland, J J Reddick, J Xi
Thiamin biosynthesis in prokaryotes.
Arch Microbiol: 1999, 171(5);293-300
[PubMed:10382260]
[WorldCat.org]
[DOI]
(P p)
Original publications