Difference between revisions of "ClpY"
m |
|||
| Line 1: | Line 1: | ||
| − | * '''Description:''' two-component ATP-dependent protease <br/><br/> | + | * '''Description:''' two-component ATP-dependent protease, ATPase subunit <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || two-component ATP-dependent protease | + | |style="background:#ABCDEF;" align="center"| '''Product''' || two-component ATP-dependent protease, <br/>ATPase subunit |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || protein degradation | |style="background:#ABCDEF;" align="center"|'''Function''' || protein degradation | ||
| Line 70: | Line 70: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' | + | * '''Interactions:''' [[ClpQ]]-[[ClpY]] {{PubMed|18689473}} |
* '''Localization:''' | * '''Localization:''' | ||
Revision as of 06:02, 15 January 2010
- Description: two-component ATP-dependent protease, ATPase subunit
| Gene name | clpY |
| Synonyms | hslU, codX |
| Essential | no |
| Product | two-component ATP-dependent protease, ATPase subunit |
| Function | protein degradation |
| MW, pI | 52 kDa, 5.31 |
| Gene length, protein length | 1401 bp, 467 aa |
| Immediate neighbours | clpQ, codY |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 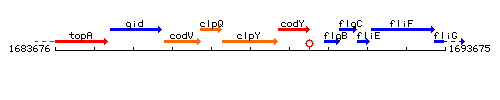
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU16160
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: HslU subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure:
- UniProt: P39778
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References