Difference between revisions of "AccD"
| Line 78: | Line 78: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/explore/explore.do?structureId=1OD2 1OD2] (carboxytransferase from yeast, equivalent to [[AccA]]-[[AccD]]) {{PubMed|12663926}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/C0SP93 C0SP93] | * '''UniProt:''' [http://www.uniprot.org/uniprot/C0SP93 C0SP93] | ||
| Line 123: | Line 123: | ||
<pubmed> 15952903 </pubmed> | <pubmed> 15952903 </pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| − | <pubmed>15066985,,14651647,16479537</pubmed> | + | <pubmed>15066985, 12663926,14651647,16479537</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:46, 6 January 2010
- Description: acetyl-CoA carboxylase (beta subunit)
| Gene name | accD |
| Synonyms | yttI |
| Essential | yes PubMed |
| Product | acetyl-CoA carboxylase (beta subunit)) |
| Function | production of malonyl-CoA, the substrate for fatty acid biosynthesis |
| MW, pI | 28 kDa, 5.344 |
| Gene length, protein length | 786 bp, 262 aa |
| Immediate neighbours | accA, ytsJ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 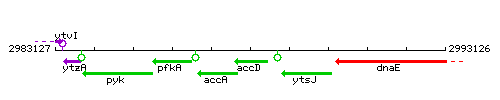
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU29210
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: C0SP93
- KEGG entry: [3]
- E.C. number: 6.4.1.2
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Stephen W White, Jie Zheng, Yong-Mei Zhang, Rock
The structural biology of type II fatty acid biosynthesis.
Annu Rev Biochem: 2005, 74;791-831
[PubMed:15952903]
[WorldCat.org]
[DOI]
(P p)
Original Publications
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Christoph Freiberg, Nina A Brunner, Guido Schiffer, Thomas Lampe, Jens Pohlmann, Michael Brands, Martin Raabe, Dieter Häbich, Karl Ziegelbauer
Identification and characterization of the first class of potent bacterial acetyl-CoA carboxylase inhibitors with antibacterial activity.
J Biol Chem: 2004, 279(25);26066-73
[PubMed:15066985]
[WorldCat.org]
[DOI]
(P p)
Virginie Molle, Masaya Fujita, Shane T Jensen, Patrick Eichenberger, José E González-Pastor, Jun S Liu, Richard Losick
The Spo0A regulon of Bacillus subtilis.
Mol Microbiol: 2003, 50(5);1683-701
[PubMed:14651647]
[WorldCat.org]
[DOI]
(P p)
Hailong Zhang, Zhiru Yang, Yang Shen, Liang Tong
Crystal structure of the carboxyltransferase domain of acetyl-coenzyme A carboxylase.
Science: 2003, 299(5615);2064-7
[PubMed:12663926]
[WorldCat.org]
[DOI]
(I p)