Difference between revisions of "NadA"
| Line 87: | Line 87: | ||
=Expression and regulation= | =Expression and regulation= | ||
| + | * '''Operon:''' ''[[nadB]]-[[nadC]]-[[nadA]]'' {{PubMed|8444804}} | ||
| − | + | * '''[[Sigma factor]]:''' [[SigA]] {{PubMed|8444804}} | |
| − | |||
| − | * '''[[Sigma factor]]:''' | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 122: | Line 121: | ||
=References= | =References= | ||
| − | <pubmed>18959769,16199587,, | + | <pubmed>18959769,16199587,,8444804, </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 20:04, 26 November 2009
- Description: quinolinate synthetase
| Gene name | nadA |
| Synonyms | |
| Essential | no |
| Product | quinolinate synthetase |
| Function | NAD biosynthesis |
| MW, pI | 41 kDa, 5.717 |
| Gene length, protein length | 1104 bp, 368 aa |
| Immediate neighbours | safA, nadC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 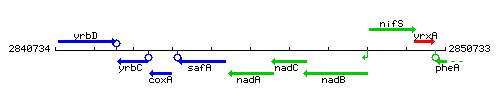
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU27850
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Glycerone phosphate + iminosuccinate = pyridine-2,3-dicarboxylate + 2 H2O + phosphate (according to Swiss-Prot)
- Protein family: Type 3 subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: spore wall (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: Q9KWZ1
- KEGG entry: [3]
- E.C. number: 2.5.1.72
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Alessandra Albertini, University of Pavia, Italy homepage
Your additional remarks
References