Difference between revisions of "TnrA"
(→Biological materials) |
(→References) |
||
| Line 132: | Line 132: | ||
<pubmed>12374841,15547269,9287005,12823818,12950915,10671441,16547045,11719184, 16547045 ,12139611, 11741602, 8799114, 12823818,15150225, 11029411,17001076,15547269, | <pubmed>12374841,15547269,9287005,12823818,12950915,10671441,16547045,11719184, 16547045 ,12139611, 11741602, 8799114, 12823818,15150225, 11029411,17001076,15547269, | ||
| − | 2573733, 8636055, 19233925, 16493705, 16885465, 6141156</pubmed> | + | 2573733, 8636055, 19233925, 16493705, 16885465, 6141156 18667567 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:35, 22 September 2009
- Description: transcriptional pleiotropic regulator invoved in global nitrogen regulation
| Gene name | tnrA |
| Synonyms | scgR |
| Essential | no |
| Product | transcription activator/ repressor |
| Function | regulation of nitrogen assimilation (positive regulation of nrgAB, nasBCDEF, gabP, ureABC, guaD; negative regulation of glnRA, gltAB) |
| Metabolic function and regulation of this protein in SubtiPathways: Nucleotides (regulation), Ile, Leu, Val, Ammonium/ glutamate, Central C-metabolism, Cell wall, Coenzyme A | |
| MW, pI | 12 kDa, 10.235 |
| Gene length, protein length | 330 bp, 110 aa |
| Immediate neighbours | mgtE, ykzB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 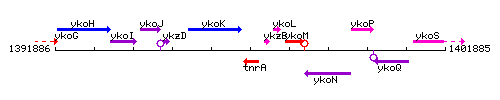
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU13310
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Genes/ operons controlled by TnrA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity: feedback-inhibited GlnA prevents TnrA from DNA binding
- Interactions: TnrA-GlnA, TnrA-NrgB PubMed, TnrA-GlnA, this interaction results in loss of TnrA DNA-binding activity
- Localization:
Database entries
- Structure:
- UniProt: Q45666
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Additional information:
Biological materials
- Mutant: GP243 (cat), GP252 (in frame deletion), available in the Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: available in the Karl Forchhammer lab
Labs working on this gene/protein
Susan Fisher, Boston, USA homepage
Your additional remarks
References
Susan H Fisher, Lewis V Wray
Novel trans-Acting Bacillus subtilis glnA mutations that derepress glnRA expression.
J Bacteriol: 2009, 191(8);2485-92
[PubMed:19233925]
[WorldCat.org]
[DOI]
(I p)
Airat Kayumov, Annette Heinrich, Margarita Sharipova, Olga Iljinskaya, Karl Forchhammer
Inactivation of the general transcription factor TnrA in Bacillus subtilis by proteolysis.
Microbiology (Reading): 2008, 154(Pt 8);2348-2355
[PubMed:18667567]
[WorldCat.org]
[DOI]
(P p)
Annette Heinrich, Kathrin Woyda, Katja Brauburger, Gregor Meiss, Christian Detsch, Jörg Stülke, Karl Forchhammer
Interaction of the membrane-bound GlnK-AmtB complex with the master regulator of nitrogen metabolism TnrA in Bacillus subtilis.
J Biol Chem: 2006, 281(46);34909-17
[PubMed:17001076]
[WorldCat.org]
[DOI]
(P p)
Susan H Fisher, Lewis V Wray
Feedback-resistant mutations in Bacillus subtilis glutamine synthetase are clustered in the active site.
J Bacteriol: 2006, 188(16);5966-74
[PubMed:16885465]
[WorldCat.org]
[DOI]
(P p)
Jill M Zalieckas, Lewis V Wray, Susan H Fisher
Cross-regulation of the Bacillus subtilis glnRA and tnrA genes provides evidence for DNA binding site discrimination by GlnR and TnrA.
J Bacteriol: 2006, 188(7);2578-85
[PubMed:16547045]
[WorldCat.org]
[DOI]
(P p)
Alain Lévine, Françoise Vannier, Cédric Absalon, Lauriane Kuhn, Peter Jackson, Elaine Scrivener, Valérie Labas, Joëlle Vinh, Patrick Courtney, Jérôme Garin, Simone J Séror
Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes.
Proteomics: 2006, 6(7);2157-73
[PubMed:16493705]
[WorldCat.org]
[DOI]
(P p)
Shigeo Tojo, Takenori Satomura, Kaori Morisaki, Ken-Ichi Yoshida, Kazutake Hirooka, Yasutaro Fujita
Negative transcriptional regulation of the ilv-leu operon for biosynthesis of branched-chain amino acids through the Bacillus subtilis global regulator TnrA.
J Bacteriol: 2004, 186(23);7971-9
[PubMed:15547269]
[WorldCat.org]
[DOI]
(P p)
Boris R Belitsky, Abraham L Sonenshein
Modulation of activity of Bacillus subtilis regulatory proteins GltC and TnrA by glutamate dehydrogenase.
J Bacteriol: 2004, 186(11);3399-407
[PubMed:15150225]
[WorldCat.org]
[DOI]
(P p)
Emmanuel Guedon, Charles M Moore, Qiang Que, Tao Wang, Rick W Ye, John D Helmann
The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons.
Mol Microbiol: 2003, 49(6);1477-91
[PubMed:12950915]
[WorldCat.org]
[DOI]
(P p)
Ken-ichi Yoshida, Hirotake Yamaguchi, Masaki Kinehara, Yo-hei Ohki, Yoshiko Nakaura, Yasutaro Fujita
Identification of additional TnrA-regulated genes of Bacillus subtilis associated with a TnrA box.
Mol Microbiol: 2003, 49(1);157-65
[PubMed:12823818]
[WorldCat.org]
[DOI]
(P p)
Jaclyn L Brandenburg, Lewis V Wray, Lars Beier, Hanne Jarmer, Hans H Saxild, Susan H Fisher
Roles of PucR, GlnR, and TnrA in regulating expression of the Bacillus subtilis ure P3 promoter.
J Bacteriol: 2002, 184(21);6060-4
[PubMed:12374841]
[WorldCat.org]
[DOI]
(P p)
Susan H Fisher, Jaclyn L Brandenburg, Lewis V Wray
Mutations in Bacillus subtilis glutamine synthetase that block its interaction with transcription factor TnrA.
Mol Microbiol: 2002, 45(3);627-35
[PubMed:12139611]
[WorldCat.org]
[DOI]
(P p)
M Wilming, K Johnsson
Inter- and intramolecular domain interactions of the catalase-peroxidase KatG from M. tuberculosis.
FEBS Lett: 2001, 509(2);272-6
[PubMed:11741602]
[WorldCat.org]
[DOI]
(P p)
L V Wray, J M Zalieckas, S H Fisher
Bacillus subtilis glutamine synthetase controls gene expression through a protein-protein interaction with transcription factor TnrA.
Cell: 2001, 107(4);427-35
[PubMed:11719184]
[WorldCat.org]
[DOI]
(P p)
B R Belitsky, L V Wray, S H Fisher, D E Bohannon, A L Sonenshein
Role of TnrA in nitrogen source-dependent repression of Bacillus subtilis glutamate synthase gene expression.
J Bacteriol: 2000, 182(21);5939-47
[PubMed:11029411]
[WorldCat.org]
[DOI]
(P p)
D Robichon, M Arnaud, R Gardan, Z Pragai, M O'Reilly, G Rapoport, M Débarbouillé
Expression of a new operon from Bacillus subtilis, ykzB-ykoL, under the control of the TnrA and PhoP-phoR global regulators.
J Bacteriol: 2000, 182(5);1226-31
[PubMed:10671441]
[WorldCat.org]
[DOI]
(P p)
L V Wray, A E Ferson, S H Fisher
Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H.
J Bacteriol: 1997, 179(17);5494-501
[PubMed:9287005]
[WorldCat.org]
[DOI]
(P p)
L V Wray, A E Ferson, K Rohrer, S H Fisher
TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis.
Proc Natl Acad Sci U S A: 1996, 93(17);8841-5
[PubMed:8799114]
[WorldCat.org]
[DOI]
(P p)
S W Brown, A L Sonenshein
Autogenous regulation of the Bacillus subtilis glnRA operon.
J Bacteriol: 1996, 178(8);2450-4
[PubMed:8636055]
[WorldCat.org]
[DOI]
(P p)
H J Schreier, S W Brown, K D Hirschi, J F Nomellini, A L Sonenshein
Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene.
J Mol Biol: 1989, 210(1);51-63
[PubMed:2573733]
[WorldCat.org]
[DOI]
(P p)
S H Fisher, A L Sonenshein
Bacillus subtilis glutamine synthetase mutants pleiotropically altered in glucose catabolite repression.
J Bacteriol: 1984, 157(2);612-21
[PubMed:6141156]
[WorldCat.org]
[DOI]
(P p)