YwlE
- Description: protein arginine phosphatase
| Gene name | ywlE |
| Synonyms | ipc-31d |
| Essential | no |
| Product | protein arginine phosphatase |
| Function | dephosphorylation of McsB |
| Gene expression levels in SubtiExpress: ywlE | |
| MW, pI | 16 kDa, 6.681 |
| Gene length, protein length | 450 bp, 150 aa |
| Immediate neighbours | ywlF, ywlD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 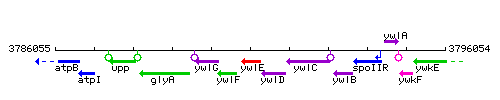
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed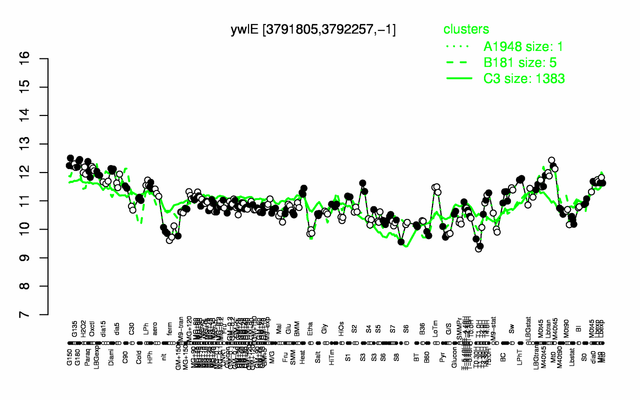
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU36930
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Protein arginine phosphate + H2O = protein arginine + phosphate PubMed
- Protein family:
- Paralogous protein(s): YfkJ
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: weak oligomerization, results in inactivation of YwlE PubMed
Database entries
- Structure: 1ZGG
- UniProt: P39155
- KEGG entry: [2]
- E.C. number: 3.1.3.48
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector: for expression, purification in E. coli with N-terminal His-tag, pRSETA available in Ulf Gerth's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: available in Ulf Gerth's lab
Labs working on this gene/protein
- Ulf Gerth, Greifswald, Germany
Your additional remarks
References
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
A K W Elsholz, K Hempel, S Michalik, K Gronau, D Becher, M Hecker, U Gerth
Activity control of the ClpC adaptor McsB in Bacillus subtilis.
J Bacteriol: 2011, 193(15);3887-93
[PubMed:21622759]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Stephan Michalik, Daniela Zühlke, Michael Hecker, Ulf Gerth
CtsR, the Gram-positive master regulator of protein quality control, feels the heat.
EMBO J: 2010, 29(21);3621-9
[PubMed:20852588]
[WorldCat.org]
[DOI]
(I p)
Jascha Blobel, Pau Bernadó, Huimin Xu, Changwen Jin, Miquel Pons
Weak oligomerization of low-molecular-weight protein tyrosine phosphatase is conserved from mammals to bacteria.
FEBS J: 2009, 276(16);4346-57
[PubMed:19678837]
[WorldCat.org]
[DOI]
(I p)
Huimin Xu, Bin Xia, Changwen Jin
Solution structure of a low-molecular-weight protein tyrosine phosphatase from Bacillus subtilis.
J Bacteriol: 2006, 188(4);1509-17
[PubMed:16452434]
[WorldCat.org]
[DOI]
(P p)
Lucia Musumeci, Cristina Bongiorni, Lutz Tautz, Robert A Edwards, Andrei Osterman, Marta Perego, Tomas Mustelin, Nunzio Bottini
Low-molecular-weight protein tyrosine phosphatases of Bacillus subtilis.
J Bacteriol: 2005, 187(14);4945-56
[PubMed:15995210]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Lucia Musumeci, Lutz Tautz, Dina Petranovic, Robert A Edwards, Peter Ruhdal Jensen, Tomas Mustelin, Josef Deutscher, Nunzio Bottini
In vitro characterization of the Bacillus subtilis protein tyrosine phosphatase YwqE.
J Bacteriol: 2005, 187(10);3384-90
[PubMed:15866923]
[WorldCat.org]
[DOI]
(P p)