YwaA
Revision as of 14:11, 13 May 2013 by 134.76.70.252 (talk)
- Description: branched-chain amino acid aminotransferase
| Gene name | ywaA |
| Synonyms | ipa-0r |
| Essential | no |
| Product | branched-chain amino acid aminotransferase |
| Function | biosynthesis of branched-chain amino acids |
| Gene expression levels in SubtiExpress: ywaA | |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis, Ile, Leu, Val | |
| MW, pI | 40 kDa, 4.952 |
| Gene length, protein length | 1089 bp, 363 aa |
| Immediate neighbours | dltE, licH |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 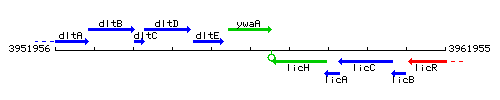
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed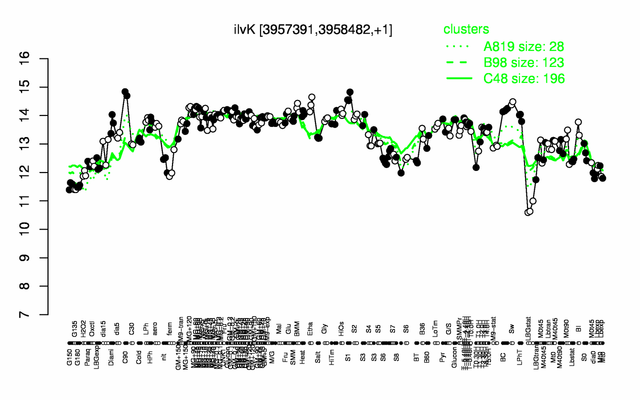
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU38550
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-leucine + 2-oxoglutarate = 4-methyl-2-oxopentanoate + L-glutamate (according to Swiss-Prot)
- Protein family: class-IV pyridoxal-phosphate-dependent aminotransferase family (according to Swiss-Prot)
- Paralogous protein(s): YbgE
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: S-cysteinylation after diamide stress (C104) PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P39576
- KEGG entry: [3]
- E.C. number: 2.6.1.42
Additional information
Expression and regulation
- Operon: ywaA PubMed
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- a ywaA::spc mutant and a bcd ybgE ywaA triple mutant are available in Linc Sonenshein's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Falko Hochgräfe, Jörg Mostertz, Dierk-Christoph Pöther, Dörte Becher, John D Helmann, Michael Hecker
S-cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress.
J Biol Chem: 2007, 282(36);25981-5
[PubMed:17611193]
[WorldCat.org]
[DOI]
(P p)
Bradley J Berger, Shane English, Gene Chan, Marvin H Knodel
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis.
J Bacteriol: 2003, 185(8);2418-31
[PubMed:12670965]
[WorldCat.org]
[DOI]
(P p)