Difference between revisions of "Spo0B"
m |
|||
| Line 103: | Line 103: | ||
* '''Mutant:''' | * '''Mutant:''' | ||
| − | **spo0B12, point mutation 85C>T (=R29W), available in [http://www.bgsc.org/ BGSC] 1S54 | + | **[http://www.ncbi.nlm.nih.gov/nuccore/254763670 spo0B12], point mutation 85C>T (=R29W), available in [http://www.bgsc.org/ BGSC] 1S54 |
| − | **spo0B136, amber mutation 103A>T (=K35X), available in [http://www.bgsc.org/ BGSC] 1S16 | + | **[http://www.ncbi.nlm.nih.gov/nuccore/254763672 spo0B136], amber mutation 103A>T (=K35X), available in [http://www.bgsc.org/ BGSC] 1S16 |
| − | **spo0B580ts, point mutation 193G>A (=E65K), available in [http://www.bgsc.org/ BGSC] 1S90 | + | **[http://www.ncbi.nlm.nih.gov/nuccore/254763674 spo0B580ts], point mutation 193G>A (=E65K), available in [http://www.bgsc.org/ BGSC] 1S90 |
| − | **spo0B581ts, point mutation 461G>A (=G154D), available in [http://www.bgsc.org/ BGSC] 1S91 | + | **[http://www.ncbi.nlm.nih.gov/nuccore/254763676 spo0B581ts], point mutation 461G>A (=G154D), available in [http://www.bgsc.org/ BGSC] 1S91 |
* '''Expression vector:''' | * '''Expression vector:''' | ||
Revision as of 14:32, 3 August 2009
- Description: sporulation initiation phosphotransferase
| Gene name | spo0B |
| Synonyms | spo0D |
| Essential | no |
| Product | sporulation initiation phosphotransferase |
| Function | initiation of sporulation |
| MW, pI | 22 kDa, 4.963 |
| Gene length, protein length | 576 bp, 192 aa |
| Immediate neighbours | obg, rpmA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 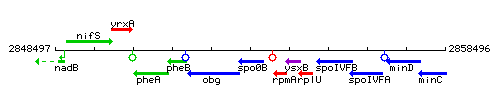
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU27930
Phenotypes of a mutant
Arrest of sporulation at stage 0 (initiation) PubMed Central
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- UniProt: P06535
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- spo0B12, point mutation 85C>T (=R29W), available in BGSC 1S54
- spo0B136, amber mutation 103A>T (=K35X), available in BGSC 1S16
- spo0B580ts, point mutation 193G>A (=E65K), available in BGSC 1S90
- spo0B581ts, point mutation 461G>A (=G154D), available in BGSC 1S91
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Kottayil I Varughese, Igor Tsigelny, Haiyan Zhao
The crystal structure of beryllofluoride Spo0F in complex with the phosphotransferase Spo0B represents a phosphotransfer pretransition state.
J Bacteriol: 2006, 188(13);4970-7
[PubMed:16788205]
[WorldCat.org]
[DOI]
(P p)
Masaya Fujita, Richard Losick
The master regulator for entry into sporulation in Bacillus subtilis becomes a cell-specific transcription factor after asymmetric division.
Genes Dev: 2003, 17(9);1166-74
[PubMed:12730135]
[WorldCat.org]
[DOI]
(P p)
Sophie J Stephenson, Marta Perego
Interaction surface of the Spo0A response regulator with the Spo0E phosphatase.
Mol Microbiol: 2002, 44(6);1455-67
[PubMed:12067336]
[WorldCat.org]
[DOI]
(P p)
J Zapf, U Sen, Madhusudan, J A Hoch, K I Varughese
A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction.
Structure: 2000, 8(8);851-62
[PubMed:10997904]
[WorldCat.org]
[DOI]
(P p)
Y L Tzeng, X Z Zhou, J A Hoch
Phosphorylation of the Spo0B response regulator phosphotransferase of the phosphorelay initiating development in Bacillus subtilis.
J Biol Chem: 1998, 273(37);23849-55
[PubMed:9726997]
[WorldCat.org]
[DOI]
(P p)
C E Grimshaw, S Huang, C G Hanstein, M A Strauch, D Burbulys, L Wang, J A Hoch, J M Whiteley
Synergistic kinetic interactions between components of the phosphorelay controlling sporulation in Bacillus subtilis.
Biochemistry: 1998, 37(5);1365-75
[PubMed:9477965]
[WorldCat.org]
[DOI]
(P p)
Y L Tzeng, J A Hoch
Molecular recognition in signal transduction: the interaction surfaces of the Spo0F response regulator with its cognate phosphorelay proteins revealed by alanine scanning mutagenesis.
J Mol Biol: 1997, 272(2);200-12
[PubMed:9299348]
[WorldCat.org]
[DOI]
(P p)
X Z Zhou, Madhusudan, J M Whiteley, J A Hoch, K I Varughese
Purification and preliminary crystallographic studies on the sporulation response regulatory phosphotransferase protein, Spo0B, from Bacillus subtilis.
Proteins: 1997, 27(4);597-600
[PubMed:9141138]
[WorldCat.org]
[DOI]
(P p)
D Burbulys, K A Trach, J A Hoch
Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay.
Cell: 1991, 64(3);545-52
[PubMed:1846779]
[WorldCat.org]
[DOI]
(P p)
U Bai, M Lewandoski, E Dubnau, I Smith
Temporal regulation of the Bacillus subtilis early sporulation gene spo0F.
J Bacteriol: 1990, 172(9);5432-9
[PubMed:2118512]
[WorldCat.org]
[DOI]
(P p)
P Zuber, R Losick
Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis.
J Bacteriol: 1987, 169(5);2223-30
[PubMed:2437099]
[WorldCat.org]
[DOI]
(P p)
F A Ferrari, K Trach, J A Hoch
Sequence analysis of the spo0B locus reveals a polycistronic transcription unit.
J Bacteriol: 1985, 161(2);556-62
[PubMed:3918016]
[WorldCat.org]
[DOI]
(P p)