Difference between revisions of "RnpA"
| Line 122: | Line 122: | ||
=References= | =References= | ||
| − | <pubmed>11454206 9563955, </pubmed> | + | <pubmed>10390342,17919279,17355991,11812129,16980484,11454206,,11454206 9563955, </pubmed> |
# Hansen, A., Pfeiffer, T., Zuleeg, T., Limmer, S., Ciesiolka, J., Feltens, R. & Hartmann, R. K. (2001). Exploring the minimal substrate requirements for trans-cleavage by RNase P holoenzyme from ''Escherichia coli'' and ''Bacillus subtilis''. Mol Microbiol 41, 131-143. [http://www.ncbi.nlm.nih.gov/sites/entrez/11454206 PubMed] | # Hansen, A., Pfeiffer, T., Zuleeg, T., Limmer, S., Ciesiolka, J., Feltens, R. & Hartmann, R. K. (2001). Exploring the minimal substrate requirements for trans-cleavage by RNase P holoenzyme from ''Escherichia coli'' and ''Bacillus subtilis''. Mol Microbiol 41, 131-143. [http://www.ncbi.nlm.nih.gov/sites/entrez/11454206 PubMed] | ||
# Stams T, Niranjanakumari S, Fierke CA, Christianson DW (1998) Ribonuclease P protein structure: evolutionary origins in the translational apparatus.''Science'' '''280:''' 752-755. [http://www.ncbi.nlm.nih.gov/sites/entrez/9563955 PubMed] | # Stams T, Niranjanakumari S, Fierke CA, Christianson DW (1998) Ribonuclease P protein structure: evolutionary origins in the translational apparatus.''Science'' '''280:''' 752-755. [http://www.ncbi.nlm.nih.gov/sites/entrez/9563955 PubMed] | ||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 09:50, 14 June 2009
- Description: protein component of ribonuclease P
| Gene name | rnpA |
| Synonyms | |
| Essential | yes PubMed |
| Product | protein component of RNase P (substrate specificity) |
| Function | cleavage of precursor sequences from the 5' ends of pre-tRNAs |
| MW, pI | 13 kDa, 10.804 |
| Gene length, protein length | 348 bp, 116 aa |
| Immediate neighbours | spoIIIJ, rpmH |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 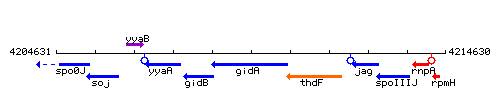
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU41050
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Endonucleolytic cleavage of RNA, removing 5'-extranucleotides from tRNA precursor (according to Swiss-Prot)
- Protein family: rnpA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Swiss prot entry: P25814
- KEGG entry: [2]
- E.C. number: 3.1.26.5
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Roland Hartmann, Marburg University, Germany homepage
Your additional remarks
References
Markus Gösringer, Roland K Hartmann
Function of heterologous and truncated RNase P proteins in Bacillus subtilis.
Mol Microbiol: 2007, 66(3);801-13
[PubMed:17919279]
[WorldCat.org]
[DOI]
(P p)
Barbara Wegscheid, Roland K Hartmann
In vivo and in vitro investigation of bacterial type B RNase P interaction with tRNA 3'-CCA.
Nucleic Acids Res: 2007, 35(6);2060-73
[PubMed:17355991]
[WorldCat.org]
[DOI]
(I p)
Markus Gössringer, Rosel Kretschmer-Kazemi Far, Roland K Hartmann
Analysis of RNase P protein (rnpA) expression in Bacillus subtilis utilizing strains with suppressible rnpA expression.
J Bacteriol: 2006, 188(19);6816-23
[PubMed:16980484]
[WorldCat.org]
[DOI]
(P p)
Christoph Rox, Ralph Feltens, Thomas Pfeiffer, Roland K Hartmann
Potential contact sites between the protein and RNA subunit in the Bacillus subtilis RNase P holoenzyme.
J Mol Biol: 2002, 315(4);551-60
[PubMed:11812129]
[WorldCat.org]
[DOI]
(P p)
A Hansen, T Pfeiffer, T Zuleeg, S Limmer, J Ciesiolka, R Feltens, R K Hartmann
Exploring the minimal substrate requirements for trans-cleavage by RNase P holoenzymes from Escherichia coli and Bacillus subtilis.
Mol Microbiol: 2001, 41(1);131-43
[PubMed:11454206]
[WorldCat.org]
[DOI]
(P p)
J M Warnecke, R Held, S Busch, R K Hartmann
Role of metal ions in the hydrolysis reaction catalyzed by RNase P RNA from Bacillus subtilis.
J Mol Biol: 1999, 290(2);433-45
[PubMed:10390342]
[WorldCat.org]
[DOI]
(P p)
T Stams, S Niranjanakumari, C A Fierke, D W Christianson
Ribonuclease P protein structure: evolutionary origins in the translational apparatus.
Science: 1998, 280(5364);752-5
[PubMed:9563955]
[WorldCat.org]
[DOI]
(P p)
- Hansen, A., Pfeiffer, T., Zuleeg, T., Limmer, S., Ciesiolka, J., Feltens, R. & Hartmann, R. K. (2001). Exploring the minimal substrate requirements for trans-cleavage by RNase P holoenzyme from Escherichia coli and Bacillus subtilis. Mol Microbiol 41, 131-143. PubMed
- Stams T, Niranjanakumari S, Fierke CA, Christianson DW (1998) Ribonuclease P protein structure: evolutionary origins in the translational apparatus.Science 280: 752-755. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed