Difference between revisions of "OdhB"
| Line 87: | Line 87: | ||
* '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P16263 P16263] | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P16263 P16263] | ||
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU19360] |
* '''E.C. number:''' [http://www.expasy.org/enzyme/2.3.1.61 2.3.1.61] 1 | * '''E.C. number:''' [http://www.expasy.org/enzyme/2.3.1.61 2.3.1.61] 1 | ||
Revision as of 03:29, 25 June 2009
- Description: 2-oxoglutarate dehydrogenase complex (dihydrolipoamide transsuccinylase, E2 subunit)
| Gene name | odhB |
| Synonyms | citM |
| Essential | yes PubMed |
| Product | 2-oxoglutarate dehydrogenase complex
(dihydrolipoamide transsuccinylase, E2 subunit) |
| Function | TCA cycle |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 45 kDa, 4.859 |
| Gene length, protein length | 1251 bp, 417 aa |
| Immediate neighbours | odhA, yocS |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 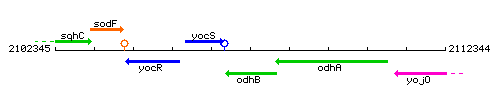
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU19360
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Succinyl-CoA + enzyme N(6)-(dihydrolipoyl)lysine = CoA + enzyme N(6)-(S-succinyldihydrolipoyl)lysine (according to Swiss-Prot)
- Protein family: sodium:bile acid symporter family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot), membrane associated PubMed
Database entries
- Structure:
- Swiss prot entry: P16263
- KEGG entry: [3]
- E.C. number: 2.3.1.61 1
Additional information
Expression and regulation
- Sigma factor: SigA
- Regulatory mechanism: CcpA: transcription repression
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
K Kobayashi, S D Ehrlich, A Albertini, G Amati, K K Andersen, M Arnaud, K Asai, S Ashikaga, S Aymerich, P Bessieres, F Boland, S C Brignell, S Bron, K Bunai, J Chapuis, L C Christiansen, A Danchin, M Débarbouille, E Dervyn, E Deuerling, K Devine, S K Devine, O Dreesen, J Errington, S Fillinger, S J Foster, Y Fujita, A Galizzi, R Gardan, C Eschevins, T Fukushima, K Haga, C R Harwood, M Hecker, D Hosoya, M F Hullo, H Kakeshita, D Karamata, Y Kasahara, F Kawamura, K Koga, P Koski, R Kuwana, D Imamura, M Ishimaru, S Ishikawa, I Ishio, D Le Coq, A Masson, C Mauël, R Meima, R P Mellado, A Moir, S Moriya, E Nagakawa, H Nanamiya, S Nakai, P Nygaard, M Ogura, T Ohanan, M O'Reilly, M O'Rourke, Z Pragai, H M Pooley, G Rapoport, J P Rawlins, L A Rivas, C Rivolta, A Sadaie, Y Sadaie, M Sarvas, T Sato, H H Saxild, E Scanlan, W Schumann, J F M L Seegers, J Sekiguchi, A Sekowska, S J Séror, M Simon, P Stragier, R Studer, H Takamatsu, T Tanaka, M Takeuchi, H B Thomaides, V Vagner, J M van Dijl, K Watabe, A Wipat, H Yamamoto, M Yamamoto, Y Yamamoto, K Yamane, K Yata, K Yoshida, H Yoshikawa, U Zuber, N Ogasawara
Essential Bacillus subtilis genes.
Proc Natl Acad Sci U S A: 2003, 100(8);4678-83
[PubMed:12682299]
[WorldCat.org]
[DOI]
(P p)
O Resnekov, L Melin, P Carlsson, M Mannerlöv, A von Gabain, L Hederstedt
Organization and regulation of the Bacillus subtilis odhAB operon, which encodes two of the subenzymes of the 2-oxoglutarate dehydrogenase complex.
Mol Gen Genet: 1992, 234(2);285-96
[PubMed:1508153]
[WorldCat.org]
[DOI]
(P p)
P Carlsson, L Hederstedt
Genetic characterization of Bacillus subtilis odhA and odhB, encoding 2-oxoglutarate dehydrogenase and dihydrolipoamide transsuccinylase, respectively.
J Bacteriol: 1989, 171(7);3667-72
[PubMed:2500417]
[WorldCat.org]
[DOI]
(P p)